Expression of Osteopontin in Gentamicin-Induced Acute Tubular Necrosis and Its Recovery Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ilias Bulaid
Ilias Bulaid Ilias Bulaid (born May 1, 1995 in Den Bosch, Netherlands) is a featherweight Ilias Bulaid Moroccan-Dutch kickboxer. Ilias was the 2016 65 kg K-1 World Tournament Runner-Up and is the current Enfusion 67 kg World Champion.[1] Born 1 May 1995 Den Bosch, As of 1 November 2018, he is ranked the #9 featherweight in the world by Netherlands [2] Combat Press. Other The Blade names Titles Nationality Dutch Moroccan 2016 K-1 World GP 2016 -65kg World Tournament Runner-Up[3] 2014 Enfusion World Champion 67 kg[4] (Three defenses; Current) Height 178 cm (5 ft 10 in) Weight 65.0 kg (143.3 lb; Professional kickboxing record 10.24 st) Division Featherweight Style Kickboxing Stance Orthodox Fighting Amsterdam, out of Netherlands Team El Otmani Gym Professional Kickboxing Record Date Result Opponent Event Location Method Round Time KO (Straight 2019- Wei Win Wu Linfeng China Right to the 3 1:11 06-29 Ninghui Body) 2018- Youssef El Decision Win Enfusion Live 70 Belgium 3 3:00 09-15 Haji (Unanimous) 2018- Hassan Wu Linfeng -67KG Loss China Decision (Split) 3 3:00 03-03 Toy Tournament Final Wu Linfeng -67KG 2018- Decision Win Xie Lei Tournament Semi China 3 3:00 03-03 (Unanimous) Finals Kunlun Fight 67 - 2017- 66kg World Sanya, Decision Win 3 3:00 11-12 Petchtanong Championship, China (Unanimous) Banchamek Quarter Finals Despite winning fight, had to withdraw from tournament due to injury. Kunlun Fight 65 - 2017- Jordan Kunlun Fight 16 Qingdao, KO (Left Body Win 1 2:25 08-27 Kranio Man Tournament China Hook) 67 kg- Final 16 KO (Spinning 2017- Manaowan Hoofddorp, Win Fight League 6 Back Kick to The 1 1:30 05-13 Netherlands Sitsongpeenong Body) 2017- Zakaria Eindhoven, Win Enfusion Live 46 Decision 5 3:00 02-18 Zouggary[5] Netherlands Defends the Enfusion -67 kg Championship. -

Oral Program November 27 (Fri) 2015 Plenary Session Noyori Conference Hall Presentation Time Title, Author(S) No
Oral Program November 27 (Fri) 2015 Plenary Session Noyori Conference Hall Presentation Time Title, Author(s) No. 14:30- Opening Ceremony 14:40 Chair: A. Katayama Opportunities from Systems and Synthetic Science for 14:40- 27-0-1 Biotechnology in Environmental Protection and Health 15:40 (Invited) Gary S. Sayler Univ. of Tennessee 15:40- Break 16:00 Chair: M. Katayama How the Smart Society will be won? Satoshi Morozumi New Energy and Industrial Technology Development Organization It is possible to understand that “Smart Society” is final goal of “Smart Community”. Also, NEDO understands that “Smart Community” is involving “Smart Suppliers” and “Smart Customers”. An important concept of “Smart Society” is allocating intelligences among public service suppliers and customers. “Smart Grid” in Electric utility business is one of the “Smart Suppliers”. In this concept, supplier side intelligences are SCADA-EMS or Distributed EMS or 16:00- 27-0-2 DMS owned by Transmission network operator or distribution network operators. Customer 17:00 (Invited) side intelligences are Home Energy Management System (HEMS), Building Energy Management System (BEMS) or Factory Energy Management System (FEMS). In the complete “Smart Society” both side intelligences are communicating with and finding best solution for each side. NEDO is promoting “Smart Community” system through several international demonstrations. In this paper, author introduces history of smart community and purpose, contents and results of current NEDO demonstration projects. 17:00- Break 17:15 17:15- Welcome Reception 19:15 Oral Program November 29 (Sun) 2015 Session 1 Room013 Minor Metal Cycles and Resource Management Presentation Time Title, Author(s) No. -

Kanazawa Medical University Research Activities and Publications VOL.30 2018 Kanazawa Medical University
Kanazawa Medical University Research Activities and Publications VOL.30 2018 Kanazawa Medical University I N D E X Code DepaertmentPage Code Depaertment Page Basic Medical Science 0475 Department of Otorhinolaryngology ・・・・・・・・ 84 0100 Department of Anatomy Ⅰ ・・・・・・・・ 1 0485 Department of Head and Neck Surgery ・・・・・・・・ 86 0110 Department of Anatomy Ⅱ ・・・・・・・・ 3 0490 Department of Dermatology ・・・・・・・・ 87 0120 Department of Physiology Ⅰ ・・・・・・・・ 5 0500 Department of Urology ・・・・・・・・ 89 0130 Department of Physiology Ⅱ ・・・・・・・・ 6 0510 Department of Obstetrics and Gynecology ・・・・・・・・ 90 0140 Department of Biochemistry Ⅰ ・・・・・・・・ 8 0520 Department of Anesthesiology and 0150 Department of Biochemistry Ⅱ ・・・・・・・・ 9 Perioperative Medicine ・・・・・・・・ 92 0160 Department of Pharmacology ・・・・・・・・ 10 0540 Department of Physical and Rehabilitation 0170 Department of Pathology Ⅰ ・・・・・・・・ 12 Medicine ・・・・・・・・ 93 0180 Department of Pathology Ⅱ ・・・・・・・・ 14 0550 Department of Emergency Medicine ・・・・・・・・ 94 0190 Department of Microbiology ・・・・・・・・ 16 0570 Department of Community Medicine ・・・・・・・・ 95 0200 Department of Immunology ・・・・・・・・ 17 0575 Department of Gastroenterological Endoscopy ・・・・・・・・ 97 0210 Department of Medical Zoology ・・・・・・・・ 18 0577 Department of Cardiovascular Intervention ・・・・・・・・ 99 0220 Department of Pathology and Laboratory 0580 Department of Oral and Maxillofacial Surgery ・・・・・・・・ 100 edicine ・・・・・・・・ 20 0590 Department of Regenerative Medicine ・・・・・・・・ 102 0230 Department of Social and Environmental Medicine ・・・・・・・・ 24 School of Nursing -
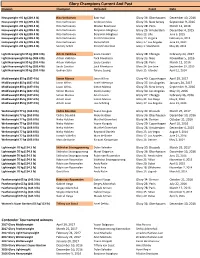
Glory Champions Current and Past Division Champion Defeated Event Date
Glory Champions Current And Past Division Champion Defeated Event Date Heavyweight +95 kg (209.4 lb) Rico Verhoeven Badr Hari Glory 36: Oberhausen December 10, 2016 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Anderson Silva Glory 33: New Jersey September 9, 2016 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Mladen Brestovac Glory 28: Paris March 12, 2016 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Benjamin Adegbuyi Glory 26: Amsterdam December 4, 2015 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Benjamin Adegbuyi Glory 22: Lille June 5, 2015 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Errol Zimmerman Glory 19: Virginia February 6, 2015 Heavyweight +95 kg (209.4 lb) Rico Verhoeven Daniel Ghiță Glory 17: Los Angeles June 21, 2014 Heavyweight +95 kg (209.4 lb) Semmy Schilt Errol Zimmerman Glory 1: Stockholm May 26, 2012 Light Heavyweight 95 kg (209.4 lb) Artem Vakhitov Saulo Cavalari Glory 38: Chicago February 24, 2017 Light Heavyweight 95 kg (209.4 lb) Artem Vakhitov Zack Mwekassa Glory 35: Nice November 5, 2016 Light Heavyweight 95 kg (209.4 lb) Artem Vakhitov Saulo Cavalari Glory 28: Paris March 12, 2016 Light Heavyweight 95 kg (209.4 lb) Saulo Cavalari Zack Mwekassa Glory 24: San Jose September 19, 2015 Light Heavyweight 95 kg (209.4 lb) Gökhan Saki Tyrone Spong Glory 15: Istanbul April 12, 2014 Middleweight 85 kg (187.4 lb) Simon Marcus Jason Wilnis Glory 40: Copenhagen April 29, 2017 Middleweight 85 kg (187.4 lb) Jason Wilnis Israel Adesanya Glory 37: Los Angeles January 20, 2017 Middleweight 85 kg (187.4 lb) Jason Wilnis Simon Marcus -

Program 1..161
The 93rd Annual Meeting of CSJ Program Chair: Ishitani, Osamu(15:30~16:20) Room S1 2S1- 06 Special Lecture Photocatalytic hydrogen evolution from water (Tokyo University of Science, Faculty of Science)Kudo, Akihiko(15:30~ 15:55) CO-LEARNING HOUSE I C102 2S1- 07 Special Lecture Development of visible light responsive semi- conductor photocatalysts(School of Engineering, The University of Tokyo) Domen, Kazunari(15:55~16:20) Frontiers of Artificial Photosynthesis JST- Chair: Inoue, Haruo(16:20~17:30) PRESTO Project "Chemical Conversion of 2S1- 08 Special Lecture How to make good use of near infrared light Light Energy" energy?(RIES, Hokkaido Univ.)Misawa, Hiroaki(16:20~16:45) 2S1- 09 Friday, March 22, AM Special Lecture Road Map of artificial photosynthesis to Future Industry(Mitsubishi Chemical Science and Technology Research Center (9:40 ~12:10) Inc.)Setoyama, Tohru(16:45~17:10) 1S1- 02# Watching Chemical Conversion of Light Energy with Picosecond 2S1- 10 Special Lecture Artificial Photosynthesis: Perspective from Time-resolved X-ray Structural Analysis(KEK)ADACHI, Shin-ichi(09:40 Science and Technology Policy and Scientists(School of Engineering, ~10:10) University of Tokyo)Hashimoto, Kazuhito(17:10~17:30) 1S1- 03# Development of Large Photofunctional Porphyrin Arrays(NAIST) ARATANI,Naoki(10:10~10:40) 1S1- 04# Development of energy conversion materials with hierarchical struc- ture using two-dimensional nanocrystals (Kyushu Univ.)IDA, Shintaro Room S2 (10:40~11:10) 1S1- 05# Development of highly-active oxygen evolving catalysts toward CO-LEARNING -

After Empire Comes Home: Economic Experiences of Japanese Civilian Repatriates, 1945-1956
The London School of Economics After empire comes home: Economic experiences of Japanese civilian repatriates, 1945-1956 Sumiyo Nishizaki A thesis submitted to the Department of Economic History of the London School of Economics for the degree of Doctor of Philosophy, London, March 2016 A part of this title is taken from Dr Lori Watt’s ground -breaking work, When Empire Comes Home. I am grateful to Dr Watt for allowing to use a phrase from her book title. Declaration I certify that the thesis I have presented for examination for the MPhil/PhD degree of the London School of Economics and Political Science is solely my own work other than where I have clearly indicated that it is the work of others (in which case the extent of any work carried out jointly by me and any other person is clearly identified in it). The copyright of this thesis rests with the author. Quotation from it is permitted, provided that full acknowledgement is made. This thesis may not be reproduced without my prior written consent. I warrant that this authorisation does not, to the best of my belief, infringe the rights of any third party. I declare that my thesis consists of 73,297 words. Statement of use of third party for editorial help (if applicable) I can confirm that my thesis was copy edited for conventions of language, spelling and grammar by Jonathan Bull, Edward Hickey, Aoi Nishizaki and Jesus Solis. 2 Acknowledgement First of all, I would like to thank to my primary supervisor Professor Janet Hunter of the Economic History Department of the London School of Economics (LSE) for her excellent supervision. -

101660 M Kawabata
Kawabata, Miki (2011) (Re)locating identities in the ancestral homeland: the complexities of belonging among the migrants from Peru in Okinawa. Mphil Thesis. SOAS, University of London http://eprints.soas.ac.uk/18464 Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non‐commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this thesis, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", name of the School or Department, PhD Thesis, pagination. (Re)locating Identities in the Ancestral Homeland: The Complexities of Belonging among the Migrants from Peru in Okinawa Thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy Miki Kawabata Department of Anthropology and Sociology School of Oriental and African Studies University of London 2011 1 Declaration for PhD thesis I have read and understood regulation 17.9 of the Regulations for students of the School of Oriental and African Studies concerning plagiarism. I undertake that all the material presented for examination is my own work and has not been written for me, in whole or in part by any other person. I also undertake that any quotation or paraphrase from the published or unpublished work of another person has been duly acknowledged in the work which I present for examination. -

For June 2013 !!
WIPU - World Independent Promoters Union specialised sanctioning body made by promoters for promoters under Oriental Pro Boxing & MMA rules in association with OPBU - Oriental Pro Boxing Union World sanctioning body for International Muaythai / K-1 & Oriental kick rules Present ORIENTAL PRO BOXING INDEPENDENT COMPUTERISED WORLD SUPER STAR RANKINGS FOR JUNE 2013 !! under rules of Thai boxing - full muaythai / International muaythai & Japanese boxing - Shootboxing / K-1 & oriental kick We have the best, unbiassed and most objective world professional fighting and ranking system ! Following suggestions from different promoters around the world, WIPU has added also 10 BEST FIGHTERS POUND FOR POUND rankings, and has included all WIPU & OPBU recognised champions into ranking system so that we can get a complete picture of best 20 fighters in the world, no matter under which fighting rules are fighting, to which promotional company or sanctioning body they belong ! Together with rankings, WIPU will publish also list of present WIPU & OPBU champions with info of upcomming title fights around the globe and those who are already confirmed or under negotiations. e-mail : [email protected] mobile phone : +385 98 421 300 www.wipu-kings.com OFFICIAL RANKINGS ENDED WITH JUNE 30th 2013 BEST TOP 10 FIGHTERS POUND – FOR - POUND 1. Yodsaenklai Fairtex ( THAI ) = 1.373 pts 2. Giorgio Petrosian ( ARM / ITA ) = 1.372 pts 3. Andy Souwer ( NL ) = 1.304 pts 4. Buakaw Banchamek ( THAI ) = 1.211 pts 5. Tyrone Spong ( SUR / NL ) = 1.209 pts 6. Semmy Schilt ( NL ) = 1.186 pts 7. Badr Hari ( MOR / NL ) = 1.159 pts 8. Kem Sitsongpeenong ( THAI ) = 1.084 pts 9. -
IMSUT Hospital Department of Advanced Medical Science 先端診療部
201 IMSUT Hospital Department of Advanced Medical Science 先端診療部 Professor Naohide Yamashita, M.D., Ph.D. 教 授 医学博士 山下直秀 Associate Professor Naoya Kato. M.D., Ph.D. 准教授(併任) 医学博士 加藤直也 Lecturer Yasuro Matsubara, M.D., Ph.D. 講師(併任) 医学博士 松原康朗 Clinical Associate Naoyuki Iso-O M.D., Ph.D. 病院講師(併任) 医学博士 磯尾直之 Clinical Associate Hideki Ohno M.D., Ph.D. 助 教 医学博士 大野秀樹 Clinical Associate Atai Watanabe M.D., Ph.D. 助 教 医学博士 渡 邉 直 Department of Advanced Medical Science was established in September 1997. Our aim is to contribute to the performance and the development of advanced therapeutic approach to the diseases. We have been participating in the poten- tially important clinical trials and the several projects in line with our principles. Our research projects are (1) Antigen-specific induction of allogeneic umbilical cord or peripheral blood-derived cytotoxic T lymphocytes, (2) Analysis of the effect of Heli- cobacter pylori eradication on non-ulcer patients, (3) Treatment of drug-resistant H. pylori infection, (4) Effect of H. pylori eradication on the expression of microR- NAs in gastric mucosa, (5) Analysis of the role of leptin in hematological malig- nancies and parallel study on using umbilical cord blood-derived cytotoxic T lym- phocytes as therapeutic strategy for hematological disorders, (6) Early diagnosis of cardiotoxicity in chemotherapy-treated patients, (7) Analysis of the potential therapeutic advantages of cell lysate from human placenta in promoting impaired cutaneous wound healing, and (8) application of 5-aminolevulinic acid for cancer therapy 1. Induction of tumor antigen-specific cytotoxic 2. -

Glory Kickboxing
GLORY KICKBOXING Glory 40: Copenhagen Copenhagen, Denmark April 29, 2017 Weight Class Winner Loser Method Round Time Middleweight Championship Middleweight 85 kg Simon Marcus © New Jason Wilnis © Decision (split) 5 3:00 Middleweight Contender Tournament Final Middleweight Yousri Belgaroui Alex Pereira Decision (unanimous) 3 3:00 Lightweight Niclas Larsen Yodkhunpon Sitmonchai Decision (unanimous) 3 3:00 Middleweight Contender Tournament Semi‐Finals Middleweight Alex Pereira Burim Rama TKO (3 knockdowns) 3 1:42 Middleweight Yousri Belgaroui Agron Preteni Decision (unanimous) 3 3:00 Superfight Series Welterweight Jamie Bates Richard Abraham Decision (unanimous) 3 3:00 Lightweight Mohammed El‐Mir Simón Santana Decision (unanimous) 3 3:00 Light Heavyweight Freddy Kemayo Imad Hadar TKO (punches) 2 2:21 Lightweight Josh Jauncey Antonio Gomez TKO (doctor stoppage) 2 1:05 Featherweight Yuhang Xie Chris Mauceri Decision (unanimous) 3 3:00 Glory 39: Brussels Brussels, Belgium March 25, 2017 Weight Class Winner Loser Method Round Time Welterweight Championship Welterweight Cedric Doumbe © Def Yoann Kongolo Decision (Unanimous) 5 3:00 Featherweight Contender Tournament Final Featherweight Petchpanomrung Kiatmookao Serhiy Adamchuk Decision (Split) 3 3:00 Heavyweight Jamal Ben Saddik Guto Inocente Decision (Unanimous) 3 3:00 Featherweight Contender Tournament Semi Final Featherweight Serhiy Adamchuk Nafi Bilalovski Decision (Unanimous) 3 3:00 Featherweight Petchpanomrung Kiatmookao Alexei Ulyanov Decision (Unanimous) 3 3:00 Lightweight Championship -

CSW2019 Program
Compound Semiconductor Week 2019 May 19 (Sun) CSW2019 Program May 19 (Sun) Special Lecture Room C 15:00-17:30 15:00 - 16:00 The Fundamentals of Quantum Dots for Advanced Photonics I Yasuhiko Arakawa The University of Tokyo, Japan Coffee Break 16:00 - 16:30 16:30 - 17:30 The Fundamentals of Quantum Dots for Advanced Photonics II Yasuhiko Arakawa The University of Tokyo, Japan Compound Semiconductor Week 2019 May 20 (Mon) May 20 (Mon) Opening Session Room A 08:30-08:40 MoPLN1 Plenary Session 1 Room A 08:40-10:00 MoPLN1-1 (Plenary) 08:40 - 09:20 GaN as a Key Material for Realizing Internet of Energy Hiroshi Amano Nagoya University, Japan MoPLN1-2 (Plenary) 09:20 - 10:00 Large-Scale Integrated Photonics for Accelerated Communication and Computing Ray Beausoleil Hewlett Packard Enterprise, United States of America Coffee Break 10:00 - 10:30 MoPLN2 Plenary Session 2 Room A 10:30-11:50 MoPLN2-1 (Plenary) 10:30 - 11:10 Bottom-Up Grown Nanowire Quantum Devices Erik Bakkers Eindhoven University of Technology, Netherlands MoPLN2-2 (Plenary) 11:10 - 11:50 Materials and Device Challenges for Next Generation LIDARS James Harris Stanford University, United States of America ISCS/IPRM Award Ceremony & Photo Room A 11:50-12:30 Lunch Break 12:30 - 14:00 Compound Semiconductor Week 2019 May 20 (Mon) MoA3 Advanced Lasers Room A 14:00-16:00 Chair: Mike Larson and Mitsuru Takenaka MoA3-1 (Invited) 14:00 - 14:30 Uncooled 53-Gbaud PAM4 Operation of EA/DFB and Directly Modulated DFB Laser for 400GbE Applications Kazuhiko Naoe,* Takayuki Nakajima, Yoshihiro Nakai, -

GOVERNMENTPRINTING AGEHOY J^^^Gh ^£D/TON'|
GOVERNMENTPRINTING AGEHOY j^^^gH ^£D/TON'| Q~+-^+-5+H $=<t*ttir EXTRA NO. 83 SUNDAY, OCTOBER 7, 1951 PUBLIC NOTICES PRIME MINISTER'S OFFICE Public Notice of Screening Result No. 49 # •E (Sept. 1-Sept. 30, 1951) October 7, 1951 Director of Cabinet Secretariat OKAZAKI Katsuo 1. This table shows the screening result made by the Prime Minister in accordance with the Imperi- al Ordinance concerning the Exclusion,Retirement, Etc. from Public Offices (Imperial Ordinance No. 1 of 1947), Ordinance concerning the Enforcement of Imperial Ordinance No. 1 of 1947 (Cabi- net and Home Ministry Ordinance No. 1 of 1947) and provisions of Cabinet Order No. 62 of 1948. 2. This table is to be most widely made public. The office of a city, ward, town or village shall, when receiving this official reports, placard the very table. This Table shall, at least, be placarded for month, and it shall, upon the next official report being received, be replaced by a new one. The old report replaced, shall not be destroyed, and be preserved after binding at the office of the city, ward town or village, to make it possible for public perusal. 3. The Questionnaires of the persons who were published on this table and of whom the screening has been completed may be offered for public perusal at the Supervisions Section of the Cabinet Secretariat or of the Offices of the Prefectures concerned. Any one may, freely peruse Questionnaires of the preceding paragraph by his request.. 4. Result (1) Number of persons subjected to screening: 6,087 Persons 1) Persons decided not coming