Review on Antimicrobial Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

35 Cyproterone Acetate and Ethinyl Estradiol Tablets 2 Mg/0
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrCYESTRA®-35 cyproterone acetate and ethinyl estradiol tablets 2 mg/0.035 mg THERAPEUTIC CLASSIFICATION Acne Therapy Paladin Labs Inc. Date of Preparation: 100 Alexis Nihon Blvd, Suite 600 January 17, 2019 St-Laurent, Quebec H4M 2P2 Version: 6.0 Control # 223341 _____________________________________________________________________________________________ CYESTRA-35 Product Monograph Page 1 of 48 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION ....................................................................... 3 SUMMARY PRODUCT INFORMATION ............................................................................................. 3 INDICATION AND CLINICAL USE ..................................................................................................... 3 CONTRAINDICATIONS ........................................................................................................................ 3 WARNINGS AND PRECAUTIONS ....................................................................................................... 4 ADVERSE REACTIONS ....................................................................................................................... 13 DRUG INTERACTIONS ....................................................................................................................... 16 DOSAGE AND ADMINISTRATION ................................................................................................ 20 OVERDOSAGE .................................................................................................................................... -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

New Insights Into the Effect of Amorolfine Nail Lacquer
Review article New insights into the effect of amorolfine nail lacquer C. Flagothier, C. Pie´ rard-Franchimont and G. E. Pie´ rard Department of Dermatopathology, University Hospital of Lie`ge, Lie`ge, Belgium Summary Despite improvements in antifungal strategies, the outcome of treating onychomycoses often remains uncertain. Several factors account for treatment failure, of which the pharmacokinetics and pharmacodynamics of the antifungal are of importance. The taxonomic nature and ungual location of the fungus cannot be neglected, besides the type of nail and its growth rate. In addition, the biological cycle of the fungus and the metabolic activity of the pathogen likely play a marked influence in drug response. The presence of natural antimicrobial peptides in the nail is also probably a key feature controlling the cure rates. There are many outstanding publications that cover the full spectrum of the field. The purpose of this review is to put in perspective some facets of activity of the topical treatment using amorolfine nail laquer. The antifungal activity of the drug is likely less pronounced in onychomycosis than that expected from conventional in vitro studies. However, the nail laquer formulation should reduce the propensity to form antifungal-resistant spores and limit the risk of reinfection. Key words: amorolfine, antifungal, fungus, onychomycosis, spore. Topical treatments are often considered to be less Introduction efficacious than current oral treatments. However, some During the last 2 decades, the efficacy of treating topical formulations may provide effects that cannot be onychomycoses has been considerably improved by achieved by other treatments. In discussing the treat- the introduction of new generations of potent antifun- ment of onychomycosis, it should not be forgotten that gals. -

Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and In-Vitro Studies
molecules Article Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and In-Vitro Studies Pradeep Kumar Bolla 1 , Carlos A. Meraz 1, Victor A. Rodriguez 1, Isaac Deaguero 1, Mahima Singh 2 , Venkata Kashyap Yellepeddi 3,4 and Jwala Renukuntla 5,* 1 Department of Biomedical Engineering, College of Engineering, The University of Texas at El Paso, 500 W University Ave, El Paso, TX 79968, USA 2 Department of Pharmaceutical Sciences, University of the Sciences in Philadelphia, Philadelphia, PA 19104, USA 3 Division of Clinical Pharmacology, Department of Pediatrics, University of Utah, Salt Lake City, UT 84112, USA 4 Department of Pharmaceutics and Pharmaceutical Chemistry, College of Pharmacy, University of Utah, Salt Lake City, UT 84112, USA 5 Department of Basic Pharmaceutical Sciences, Fred Wilson School of Pharmacy, High Point University, High Point, NC 27240, USA * Correspondence: [email protected] Received: 9 August 2019; Accepted: 28 August 2019; Published: 29 August 2019 Abstract: Global incidence of superficial fungal infections caused by dermatophytes is high and affects around 40 million people. It is the fourth most common cause of infection. Clotrimazole, a broad spectrum imidazole antifungal agent is widely used to treat fungal infections. Conventional topical formulations of clotrimazole are intended to treat infections by effective penetration of drugs into the stratum corneum. However, drawbacks such as poor dermal bioavailability, poor penetration, and variable drug levels limit the efficiency. The present study aims to load clotrimazole into ufosomes and evaluate its topical bioavailability. Clotrimazole loaded ufosomes were prepared using cholesterol and sodium oleate by thin film hydration technique and evaluated for size, polydispersity index, and entrapment efficiency to obtain optimized formulation. -
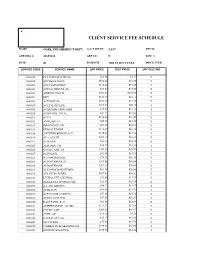
Oakland Sheriff's Dept Fee Schedule 02.09
CLIENT SERVICE FEE SCHEDULE NAME: OAKLAND SHERIFF'S DEPT ACCT SETUP: 2/1/87 REP ID: GRP BILL #: 22857610 GRP CD: N DISC %: FS ID: 01 FS DESCR: 2003 CLIENT FEES PRICE TYPE: SERVICE CODE SERVICE NAME UPF PRICE TEST PRICE UPF DISC IND 0000018 PLT SODIUM CITRATE $12.86 $4.37 Y 0000022 APC RESISTANCE $101.62 $34.55 Y 0000201 ACETAMINOPHEN $110.24 $37.48 Y 0000202 ACETALDEHYDE (B) $73.00 $73.00 N 0000203 ARSENIC,TISSUE $302.00 $302.00 N 0000204 DHT $182.70 $62.12 Y 0000205 ACETONE (B) $102.20 $34.75 Y 0000206 ACETYLCHOLINE $165.00 $56.10 Y 0000208 ACID PHOS, PROS, IMM $35.60 $12.10 Y 0000210 ACID PHOS, TOTAL $37.22 $12.65 Y 0000211 ACTH $110.00 $37.40 Y 0000212 AMYLASE (U) $45.45 $15.45 Y 0000213 IMMUNOFIX, UR $67.39 $22.91 Y 0000214 ETHOSUXIMIDE $112.07 $38.10 Y 0000216 ANTITHROMBIN III ACT $170.46 $57.96 Y 0000219 ALA, QUANT $107.10 $36.41 Y 0000223 ALBUMIN $22.88 $22.88 N 0000224 ALBUMIN, CSF $36.25 $12.33 Y 0000225 CYCLIC AMP, UR $205.80 $69.97 Y 0000227 ALDOLASE $32.08 $10.91 Y 0000228 A-2-MACROGLOB. $78.75 $26.78 Y 0000229 ALDOSTERONE (U) $251.58 $85.54 Y 0000230 ALDOSTERONE $207.10 $70.41 Y 0000231 ALK PHOS ISOENZYMES $61.34 $20.86 Y 0000232 AFP, FLUID W/RFX $107.40 $36.52 Y 0000233 LEUKOCYTE ALK PHOS $34.60 $11.76 Y 0000234 ALKALINE PHOSPHATASE $22.88 $22.88 N 0000235 A-1-ANTITRYPSN $40.22 $13.67 Y 0000236 AMIKACIN $99.91 $33.97 Y 0000237 AFP,TUMOR (CHIRON) $53.86 $18.31 Y 0000238 AMINO ACID SCR $87.15 $29.63 Y 0000240 RAST PANEL #103 $62.00 $62.00 N 0000241 AMPHETAMINE GC/MS $111.70 $37.98 Y 0000242 CYCLIC AMP $205.80 $69.97 Y 0000243 AMYLASE $16.42 $5.58 Y 0000248 HACKBERRY IGE $35.40 $35.40 N 0000249 ANA W/RFX $55.00 $18.70 Y 0000250 *AMIKACIN,PEAK&TROUGH $199.83 $67.94 Y 0000251 ANDROSTENEDIONE $180.65 $61.42 Y 0000252 ANTI-DIUR. -

The Management of Common Skin Conditions in General Practice
Management of Common Skin Conditions In General Practice including the “red rash made easy” © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care University of Auckland, Tamaki Campus Reviewed by Hon A/Prof Amanda Oakley - 2019 http://www.dermnetnz.org Management of Common Skin Conditions In General Practice Contents Page Derm Map 3 Classic location: infants & children 4 Classic location: adults 5 Dermatology terminology 6 Common red rashes 7 Other common skin conditions 12 Common viral infections 14 Common bacterial infections 16 Common fungal infections 17 Arthropods 19 Eczema/dermatitis 20 Benign skin lesions 23 Skin cancers 26 Emergency dermatology 28 Clinical diagnosis of melanoma 31 Principles of diagnosis and treatment 32 Principles of treatment of eczema 33 Treatment sequence for psoriasis 34 Topical corticosteroids 35 Combination topical steroid + antimicrobial 36 Safety with topical corticosteroids 36 Emollients 37 Antipruritics 38 For further information, refer to: http://www.dermnetnz.org And http://www.derm-master.com 2 © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care, University of Auckland, Tamaki Campus. Management of Common Skin Conditions In General Practice DERM MAP Start Is the patient sick ? Yes Rash could be an infection or a drug eruption? No Insect Bites – Crop of grouped papules with a central blister or scab. Is the patient in pain or the rash Yes Infection: cellulitis / erysipelas, impetigo, boil is swelling, oozing or crusting? / folliculitis, herpes simplex / zoster. Urticaria – Smooth skin surface with weals that evolve in minutes to hours. No Is the rash in a classic location? Yes See our classic location chart . -

Butenafine Hydrochloride/Climbazole 529 to Be Reduced in Patients with Hepatic Impairment (See Profile Solution in Water Has a Ph of 8.0 to 9.0
Butenafine Hydrochloride/Climbazole 529 to be reduced in patients with hepatic impairment (see Profile solution in water has a pH of 8.0 to 9.0. Protect from light. below). Chlormidazole hydrochloride is an imidazole antifungal used USP 31 (Ciclopirox Olamine). A white to slightly yellowish- topically as the hydrochloride in the treatment of fungal infec- white, crystalline powder. Slightly soluble in water; very soluble ◊ Reviews. tions of the skin. in alcohol and in dichloromethane; practically insoluble in cy- 1. Letscher-Bru V, Herbrecht R. Caspofungin: the first representa- For a discussion of the caution needed when using azole antifun- clohexane. pH of a 1% solution in water is between 8.0 and 9.0. tive of a new antifungal class. J Antimicrob Chemother 2003; 51: gals during pregnancy, see under Pregnancy in Precautions of Store in airtight containers at a temperature of 5° to 25°. Protect 513–21. Fluconazole, p.532. from light. 2. Deresinski SC, Stevens DA. Caspofungin. Clin Infect Dis 2003; 36: 1445–57. Preparations Adverse Effects 3. Denning DW. Echinocandin antifungal drugs. Lancet 2003; 362: Proprietary Preparations (details are given in Part 3) Irritation and pruritus have been reported after topical applica- 1142–51. Pol.: Unifungicid. tion of ciclopirox. 4. McCormack PL, Perry CM. Caspofungin: a review of its use in the treatment of fungal infections. Drugs 2005; 65: 2049–68. Multi-ingredient: Austria: Myco-Synalar; Pol.: Polfungicid; Switz.: Antimicrobial Action 5. Morris MI, Villmann M. Echinocandins in the management of Myco-Synalar†. Ciclopirox has a wide spectrum of antifungal activity. It inhibits invasive fungal infections, part 1. -

Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020
First independent framework for assessing pharmaceutical company action Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020 ACKNOWLEDGEMENTS The Access to Medicine Foundation would like to thank the following people and organisations for their contributions to this report.1 FUNDERS The Antimicrobial Resistance Benchmark research programme is made possible with financial support from UK AID and the Dutch Ministry of Health, Welfare and Sport. Expert Review Committee Research Team Reviewers Hans Hogerzeil - Chair Gabrielle Breugelmans Christine Årdal Gregory Frank Fatema Rafiqi Karen Gallant Nina Grundmann Adrián Alonso Ruiz Hans Hogerzeil Magdalena Kettis Ruth Baron Hitesh Hurkchand Joakim Larsson Dulce Calçada Joakim Larsson Marc Mendelson Moska Hellamand Marc Mendelson Margareth Ndomondo-Sigonda Kevin Outterson Katarina Nedog Sarah Paulin (Observer) Editorial Team Andrew Singer Anna Massey Deirdre Cogan ACCESS TO MEDICINE FOUNDATION Rachel Jones The Access to Medicine Foundation is an independent Emma Ross non-profit organisation based in the Netherlands. It aims to advance access to medicine in low- and middle-income Additional contributors countries by stimulating and guiding the pharmaceutical Thomas Collin-Lefebvre industry to play a greater role in improving access to Alex Kong medicine. Nestor Papanikolaou Address Contact Naritaweg 227-A For more information about this publication, please contact 1043 CB, Amsterdam Jayasree K. Iyer, Executive Director The Netherlands [email protected] +31 (0) 20 215 35 35 www.amrbenchmark.org 1 This acknowledgement is not intended to imply that the individuals and institutions referred to above endorse About the cover: Young woman from the Antimicrobial Resistance Benchmark methodology, Brazil, where 40%-60% of infections are analyses or results. -

Topical and Systemic Antifungal Therapy for Chronic Rhinosinusitis (Protocol)
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of East Anglia digital repository Cochrane Database of Systematic Reviews Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Head K, Sacks PL, Chong LY, Hopkins C, Philpott C Head K, Sacks PL, Chong LY, Hopkins C, Philpott C. Topical and systemic antifungal therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No.: CD012453. DOI: 10.1002/14651858.CD012453. www.cochranelibrary.com Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 BACKGROUND .................................... 1 OBJECTIVES ..................................... 3 METHODS ...................................... 3 ACKNOWLEDGEMENTS . 8 REFERENCES ..................................... 9 APPENDICES ..................................... 10 CONTRIBUTIONSOFAUTHORS . 25 DECLARATIONSOFINTEREST . 26 SOURCESOFSUPPORT . 26 NOTES........................................ 26 Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) i Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. [Intervention Protocol] Topical and systemic antifungal therapy for chronic rhinosinusitis Karen Head1, Peta-Lee Sacks2, Lee Yee Chong1, Claire Hopkins3, Carl Philpott4 1UK Cochrane Centre, -

Diagnosis and Treatment of Tinea Versicolor Ronald Savin, MD New Haven, Connecticut
■ CLINICAL REVIEW Diagnosis and Treatment of Tinea Versicolor Ronald Savin, MD New Haven, Connecticut Tinea versicolor (pityriasis versicolor) is a common imidazole, has been used for years both orally and top superficial fungal infection of the stratum corneum. ically with great success, although it has not been Caused by the fungus Malassezia furfur, this chronical approved by the Food and Drug Administration for the ly recurring disease is most prevalent in the tropics but indication of tinea versicolor. Newer derivatives, such is also common in temperate climates. Treatments are as fluconazole and itraconazole, have recently been available and cure rates are high, although recurrences introduced. Side effects associated with these triazoles are common. Traditional topical agents such as seleni tend to be minor and low in incidence. Except for keto um sulfide are effective, but recurrence following treat conazole, oral antifungals carry a low risk of hepato- ment with these agents is likely and often rapid. toxicity. Currently, therapeutic interest is focused on synthetic Key Words: Tinea versicolor; pityriasis versicolor; anti “-azole” antifungal drugs, which interfere with the sterol fungal agents. metabolism of the infectious agent. Ketoconazole, an (J Fam Pract 1996; 43:127-132) ormal skin flora includes two morpho than formerly thought. In one study, children under logically discrete lipophilic yeasts: a age 14 represented nearly 5% of confirmed cases spherical form, Pityrosporum orbicu- of the disease.3 In many of these cases, the face lare, and an ovoid form, Pityrosporum was involved, a rare manifestation of the disease in ovale. Whether these are separate enti adults.1 The condition is most prevalent in tropical tiesN or different morphologic forms in the cell and semitropical areas, where up to 40% of some cycle of the same organism remains unclear.: In the populations are affected. -

THE INFLUENCE of SOME ANTIFUNGAL ANTIBIOTICS on NEUROMUSCULAR TRANSMISSION B.L
THE INFLUENCE OF SOME ANTIFUNGAL ANTIBIOTICS ON NEUROMUSCULAR TRANSMISSION B.l M. SIRSI Pharmacology Laboratory, Indian Institute of Science, Bangalore, 12. (Received January 25, 1963) The influcncc of the polyene antifungal antibiotics pimaricin, amphotericin A and B and nystatin, on neuromuscular transmission has been studied. In presence of these antibiotics both direct and indirect electrical stimulation is found. to cause varying degrees of contracture and diminished excitability of the musculature. This is in contrast to the effect of Hamycin, another polyene antibiotic which produces loss of excitability without any initial contracture of the diaphragm. Certain antibiotics have been shown to produce neuromuscular block, both in experimental animals and in humaps. Intraperitoneal administra- tion of neomycin has been followed by respiratory insufficiency or apnoea; streptomycin has caused neuromuscular block in experimental animals and in interccstal nerve preparations of human and is strongly incriminated in causing the muscular weakness and visual difficulty as also post-operative paralysis by neuromuscular blockage (Fisk, 1961, Bush, 1961). Polymyxin B is also reported to exhibit blocking action on the muscle end plate (Sabawala and Dillon, 1959). The following communication deals with the effect of certain antifungal antibiotics on the neuromuscular junction as studied by the rat phrenic nerve diaphragm preparations. METHODS The antibiotics studied were, pimaricin, amphotericin A and B, and nystatin. Their effects have been compared with those of hamycin reported earlier (Sirsi, 1963). -- The required concentrations of the antibiotics were prepared in propylene glycol and further dilutions in water. Phrenic nerve diaphragm preparations were made as described by Bulbring (1916) and were suspended in abath of 75 ml of aerated Tyrode's solution which contained twice the amount of glucose as stated in original M. -

Step Therapy Criteria Drug Class Topical Antifungal Agents (Brand Products Only)
STEP THERAPY CRITERIA DRUG CLASS TOPICAL ANTIFUNGAL AGENTS (BRAND PRODUCTS ONLY) BRAND NAME (generic) ECOZA (econazole) ERTACZO (sertaconazole) EXELDERM (sulconazole nitrate) LOPROX (ciclopirox shampoo) LOTRISONE (clotrimazole/betamethasone) LUZU (luliconazole) MENTAX (butenafine) NAFTIN (naftifine) OXISTAT (oxiconazole) VUSION (miconazole/zinc oxide/white petrolatum) XOLEGEL (ketoconazole) Antifungal Topical Step Therapy Policy 06-2017 CVS Caremark is an independent company that provides pharmacy benefit management services to CareFirst BlueCross BlueShield and CareFirst BlueChoice, Inc. members. CareFirst BlueCross BlueShield is the shared business name of CareFirst of Maryland, Inc. and Group Hospitalization and Medical Services, Inc. CareFirst of Maryland, Inc., Group Hospitalization and Medical Services, Inc., CareFirst BlueChoice, Inc., The Dental Network and First Care, Inc. are independent licensees of the Blue Cross and Blue Shield Association. In the District of Columbia and Maryland, CareFirst MedPlus is the business name of First Care, Inc. In Virginia, CareFirst MedPlus is the business name of First Care, Inc. of Maryland (used in VA by: First Care, Inc.). ® Registered trademark of the Blue Cross and Blue Shield Association Page 1 of 4 Status: CVS Caremark Criteria Type: Initial Step Therapy; Post Step Therapy Prior Authorization POLICY FDA-APPROVED INDICATIONS Ecoza Ecoza topical 1% foam is indicated for the treatment of interdigital tinea pedis caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton