How to Do Film and Digital Imaging of Microscopic Preparations Namboori B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Megaplus Conversion Lenses for Digital Cameras
Section2 PHOTO - VIDEO - PRO AUDIO Accessories LCD Accessories .......................244-245 Batteries.....................................246-249 Camera Brackets ......................250-253 Flashes........................................253-259 Accessory Lenses .....................260-265 VR Tools.....................................266-271 Digital Media & Peripherals ..272-279 Portable Media Storage ..........280-285 Digital Picture Frames....................286 Imaging Systems ..............................287 Tripods and Heads ..................288-301 Camera Cases............................302-321 Underwater Equipment ..........322-327 PHOTOGRAPHIC SOLUTIONS DIGITAL CAMERA CLEANING PRODUCTS Sensor Swab — Digital Imaging Chip Cleaner HAKUBA Sensor Swabs are designed for cleaning the CLEANING PRODUCTS imaging sensor (CMOS or CCD) on SLR digital cameras and other delicate or hard to reach optical and imaging sur- faces. Clean room manufactured KMC-05 and sealed, these swabs are the ultimate Lens Cleaning Kit in purity. Recommended by Kodak and Fuji (when Includes: Lens tissue (30 used with Eclipse Lens Cleaner) for cleaning the DSC Pro 14n pcs.), Cleaning Solution 30 cc and FinePix S1/S2 Pro. #HALCK .........................3.95 Sensor Swabs for Digital SLR Cameras: 12-Pack (PHSS12) ........45.95 KA-11 Lens Cleaning Set Includes a Blower Brush,Cleaning Solution 30cc, Lens ECLIPSE Tissue Cleaning Cloth. CAMERA ACCESSORIES #HALCS ...................................................................................4.95 ECLIPSE lens cleaner is the highest purity lens cleaner available. It dries as quickly as it can LCDCK-BL Digital Cleaning Kit be applied leaving absolutely no residue. For cleaing LCD screens and other optical surfaces. ECLIPSE is the recommended optical glass Includes dual function cleaning tool that has a lens brush on one side and a cleaning chamois on the other, cleaner for THK USA, the US distributor for cleaning solution and five replacement chamois with one 244 Hoya filters and Tokina lenses. -

Instructions for Use with Nikon Coolpix 5400 Quick Start Guide
0-360 Panoramic Optic Setup for Nikon Coolpix 5400 1) Mount Camera to tripod, securely, with lens pointing vertically. A note about Aperture, Depth of Field, and Field of View 2) Thread 0-360 Panoramic Optic (with thread adapter) to camera. (Do not overtighten.) Aperture- a mechanism behind the camera lens similar to the iris of your eye, 3) Adjust tripod until Optic is vertical (refer to bubble level on top of Optic). opening and closing to adjust the amount of light entering the camera. The aperture 4) Turn Power on, and set Mode to "A" (Aperture Priority) opening also determines the Depth of Field of the image. Depth of Field- describes the objects in the image which are in focus, in terms of 5) First-time setup: Press Menu Button and set the following: their distance from the camera. For example, a camera focused at 30m, with a Depth Focus Options > AF Area Mode > Manual of Field of 8m, will have objects from 26-34m from the camera in focus. Objects Focus Options > Auto Focus Mode > Single closer than 26m or further than 34m will start to become blurry. Field of View- the vertical Field of View (vFOV) of the 0-360 attachment. The 0-360 Speedlight options > Speedlight Control > Internal Off has a vFOV of 115 degrees, meaning it will “see” from 52.5º above the horizon to 62.5º Image Quality/Size > FINE, 2592x1944 below the horizon. ISO > 100 A smaller Aperture opening (higher F-Stop number) allows less light to enter the 6) Rotate Command Dial until "F8.0" appears on screen. -

Sept.News 2003
BEAU PHOTO SUPPLIES INC. 1520 W. 6TH AVE. PHONE: (604) 734 7771 VANCOUVER, BC FAX: (604) 734 7730 CANADA V6J 1R2 www.beauphoto.com LAMINATES UP TO 70% OFF !!! SALE ON NEW OVERSTOCK LAMINATES! Canvas rolls Regular $187.00 Sale ! $99.50 Matte Smooth 8x22 sheets (200) Regular $130.00 Sale ! $79.50 CLEARANCE on slightly flawed, open laminate boxes! Regular $219.00 12”x15” sheets (minimum 150 sheets per box) 9”x22” sheets (minimum 150 sheets per box) ONLY $49.95 ea full and almost full rolls Variety of finishes available - canvas, matte smooth, leather, small texture As is. All sales final. While quantities last! News from the back !!! For those who have been waiting, you already know! For those of you who haven’t, it has finally been announced! Nikon’s next generation Professional Digital SLR for photojournalism, action and sports. The new D2H incorporates an amazing combination of speed, resolution, handling and faster workflow for total image quality and performance. As for specs ? Check these out: 8 fps for up to 40 consecutive JPEG or 25 RAW (NEF) full resolution (2,464 x 1,632 pixel) images. The D2H also has a very short 37ms shutter time lag. Instant power up means the D2H is ready to go when you are. Nikon’s exclusive new 4.1 mp imaging sensor improves on power consumption and minimize dark noise all while increasing speed and image quality. This new sensor is also built to read 2 channels of data from each pixel at once for maximum speed in image reproduction. -
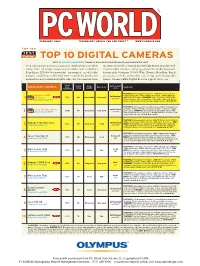
TOP 10 DIGITAL CAMERAS Visit Find.Pcworld.Com/39623 for Reviews of All Products Tested This Month and Ranked in This Chart
FEBRUARY 2004 TECHNOLOGY ADVICE YOU CAN TRUST TM WWW.PCWORLD.COM TOP 100 TEST Center TOP 10 DIGITAL CAMERAS Visit find.pcworld.com/39623 for reviews of all products tested this month and ranked in this chart. our advanced digital cameras chart covers a lot of ter- an array of creative controls not typically found in point-and- ritory, from relatively inexpensive models such as Kodak’s shoot models. Excellent imaging earned the modestly priced, EasyShare DX6490 to powerful “prosumer” models like feature-rich Olympus C-5060 Wide Zoom a Best Buy. But if Canon’s single-lens reflex EOS 10D, suitable for profession- you want an SLR camera that can accept interchangeable al shooters and serious hobbyists alike. All ten cameras share lenses, Canon’s EOS Digital Rebel is a great value, too. Street Tested price Street (12/8/00)Overall Image Battery life/ ADVANCED CAMERA price Ease of use Comments 1 (12/01/03) rating quality shots Best Chart FEATURES: 5.1-megapixel resolution, 32MB XD-Picture Card and 1 BUY Olympus C-5060 Text 2 Outstanding/ CompactFlash slot, 27mm to 110mm focal range, 640 by 480 video Best NEW $700 86 Outstanding Very good with audio, 17.9 ounces. SUMMARY: Successor to the C-5050, this 1 BUY Wide Zoom over 500 2 find.pcworld.com/39527 bulky model has a 4X zoom in place of the older model’s 3X. Has in- tuitive controls and earned top image-quality scores. (11112) Best FEATURES: 6.3-megapixel resolution, CompactFlash slot (media not Mon 00 2 BUY Outstanding/ included), 28mm to 90mm focal range, no video or audio recording, Best Canon EOS Digital Rebel $1000 85 Very good Very good 29.5 ounces. -

Underwater Quick Dial VIDEO HOUSINGS, MONITORS & DIVE LIGHTS 821 UNDERWATER
Underwater Quick Dial VIDEO HOUSINGS, MONITORS & DIVE LIGHTS 821 UNDERWATER Atomic Solar Watch Camcorder Housings 2.5” Internal Color Monitor $12995 GW700A Tested for use to a depth of 33’ (10m). $19995 Remarkably light and still robust, they V2000 • Atomic Timekeeping • Tough Solar Power provide effective against sand, dust, with Mini 1/8” Plug • Shock Resistant • 200M Water Resistant rain, seawater, mud, humidity, etc. #25CMM18 • Auto EL Backlight with Afterglow for Sony: • Time Recorder (30 Records) Most TR...................#VST ....264.95 NEW! for Sony FX1 with RCA Plug Most TRV...........#VDX100 ....344.95 • World Times (30 Cities) TRV-8, 10................#VDS ....274.95 #VFX.....................499.95 #25CMRCA • 12/24-Hour Formats TRV-950 ..................#VST ....264.95 for JVC DV-1 ...........#VJD ....269.95 • 1/100 Second Stopwatch PC-1, 7, 10 .............#VPC ....274.95 for Canon Optura ...#VMV ....344.95 • 4 Daily Alarms & 1 Snooze Alarm PC-6, 8, 9, 101 .......#VPD ....274.95 for Canon ZR Series.#VDS ....274.95 PC-115, 120............#VVX ....354.95 for Canon Elura ..#VMV20 ....374.95 • For use with All • Hourly Time Signal • Auto-Calendar VX-1000...............#V1000 ....449.95 for Canon GL-2 ....#VXM2 ....434.95 Pro-Pak 6 and • Battery Power Indicator • Power Saving Function VX2000 ................#V2000 ....479.95 for Canon XL-1 ........#VXL ....949.95 Pro-Pak 8 Housings Video Housings Video Housings 3.5” TFT LCD Color Monitor #ACFM0350 Dive Buddy Plus Housings PC350 Housing $ 95 • Depth Rated to 330’ (100m) for Sony -

This Item Was Submitted to Loughborough's Institutional
This item was submitted to Loughborough’s Institutional Repository (https://dspace.lboro.ac.uk/) by the author and is made available under the following Creative Commons Licence conditions. For the full text of this licence, please go to: http://creativecommons.org/licenses/by-nc-nd/2.5/ SPATIAL MEASUREMENT WITH CONSUMER GRADE DIGITAL CAMERAS Ren´eWackrow Department of Civil and Building Engineering Loughborough University A Doctoral Thesis Submitted for the Degree of Doctor of Philosophy October 2008 c by Ren´eWackrow (2008) Abstract Key words: photogrammetry, camera calibration, digital camera, camera stability, image configuration, spatial measurement, digital elevation model, lens distortion This thesis develops the potential of consumer-grade digital cameras for accu- rate spatial measurement. These cameras are generally considered unstable but their uncertain geometry can be partially resolved by calibration. The valid- ity of calibration data over time should be carefully assessed before subsequent photogrammetric measurement. The use of such digital cameras for photogram- metric measurement is increasingly accepted in many industrial fields but also in a diverse range of fields including medical and forensic science and architectural work. However, the stability of these cameras is less frequently reported in the literature, which can be attributed to the absence of standards for quantitative analyses of camera stability. The approach used to assess camera stability in this study is based on compar- ing the accuracy in the reconstructed object space, achieved using sets of interior orientation parameters of a sensor, derived in different calibration sessions. This technique was successfully applied to assess the temporal stability and manufac- turing consistency of seven identical Nikon Coolpix 5400 digital cameras. -

THE ASSESSMENT of HIGH DYNAMIC RANGE LUMINANCE MEASUREMENTS with LED LIGHTING Yulia I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Architectural Engineering -- Dissertations and Architectural Engineering Student Research 4-2012 THE ASSESSMENT OF HIGH DYNAMIC RANGE LUMINANCE MEASUREMENTS WITH LED LIGHTING Yulia I. Tyukhova University of Nebraska – Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/archengdiss Part of the Architectural Engineering Commons Tyukhova, Yulia I., "THE ASSESSMENT OF HIGH DYNAMIC RANGE LUMINANCE MEASUREMENTS WITH LED LIGHTING" (2012). Architectural Engineering -- Dissertations and Student Research. 17. http://digitalcommons.unl.edu/archengdiss/17 This Article is brought to you for free and open access by the Architectural Engineering at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Architectural Engineering -- Dissertations and Student Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. THE ASSESSMENT OF HIGH DYNAMIC RANGE LUMINANCE MEASUREMENTS WITH LED LIGHTING by Yulia I. Tyukhova A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Architectural Engineering Under the Supervision of Professor Clarence Waters Lincoln, Nebraska April, 2012 THE ASSESSMENT OF HIGH DYNAMIC RANGE LUMINANCE MEASUREMENTS WITH LED LIGHTING Yulia I. Tyukhova, M.S. University of Nebraska, 2012 Adviser: Clarence Waters This research investigates whether a High Dynamic Range Imaging (HDRI) technique can accurately capture luminance values of a single LED chip. Previous studies show that a digital camera with exposure capability can be used as a luminance mapping tool in a wide range of luminance values with an accuracy of 10%. Previous work has also demonstrated the ability of HDRI to capture a rapidly- changing lighting environment with the sun. -

1 Vegetation Water Content During SMEX04 from Ground Data And
1 Vegetation water content during SMEX04 from ground data and Landsat 5 Thematic Mapper imagery M. Tugrul Yilmaz a, E. Raymond Hunt, Jr. a, *, Lyssa D. Goins b, Susan L. Ustin c, Vern C. Vanderbilt d, Thomas J. Jackson a a USDA Agricultural Research Service, Hydrology and Remote Sensing Laboratory, Beltsville MD, USA b Soil, Water and Environmental Sciences, University of Arizona, Tucson AZ, USA c Department of Land Air and Water Resources, University of California, Davis CA, USA d NASA Ames Research Center, Moffett Field CA, USA * Corresponding author. USDA ARS HRSL, Building 007, Room 104, BARC-West, 10300 Baltimore Avenue, Beltsville, MD 20705-2350, USA; Tel.:+1-301-504-5278; fax: +1-301-504-8931. E-mail address : [email protected] (E. R. Hunt, Jr.) ____________________________________________________________________ 2 Abstract Vegetation water content is an important parameter for retrieval of soil moisture from microwave data and for other remote sensing applications. Because liquid water absorbs in the shortwave infrared, the normalized difference infrared index (NDII), calculated from Landsat 5 Thematic Mapper band 4 (0.76-0.90 :m wavelength) and band 5 (1.55-1.65 :m wavelength), can be used to determine canopy equivalent water thickness (EWT), which is defined as the water volume per leaf area times the leaf area index (LAI). Alternatively, average canopy EWT can be determined using a landcover classification, because different vegetation types have different average LAI at the peak of the growing season. The primary contribution of this study for the Soil Moisture Experiment 2004 was to sample vegetation for the Arizona and Sonora study areas. -

Contax Tvs Digital
Section1 PHOTO - VIDEO - PRO AUDIO Digital Still Cameras Introduction to Digital Imaging ...8-11 Canon..............................................12-28 Casio................................................29-31 Contax.............................................32-33 Fuji...................................................34-45 Hewlett Packard ...........................46-49 Kodak..............................................50-53 Kyocera...........................................54-57 Leica................................................58-59 Minolta............................................60-75 Nikon...............................................76-87 Olympus .......................................88-101 Panasonic...................................102-109 Pentax.........................................110-115 Samsung.............................................116 Sony ............................................117-136 Vivitar ................................................137 INTRODUCTION TO Digital Imaging—a primer for the uninitiated Digital imaging is exploding. It seems everybody is doing it. And you think you want to get into it. Fine, but where do you start? What equipment do you need? No problem. But before we get to that, let us begin with a little background information so you’ll have a better understand of what digital imaging is. Until about five years ago imaging was an analog affair. Pictures were “recorded” onto film and the manipulation of images took place in a darkroom—a messy, wasteful and environmentally unfriendly -

Interpreting Canopy Development and Physiology Using a European Phenology Camera Network at flux Sites
Biogeosciences, 12, 5995–6015, 2015 www.biogeosciences.net/12/5995/2015/ doi:10.5194/bg-12-5995-2015 © Author(s) 2015. CC Attribution 3.0 License. Interpreting canopy development and physiology using a European phenology camera network at flux sites L. Wingate1, J. Ogée1, E. Cremonese2, G. Filippa2, T. Mizunuma3, M. Migliavacca4, C. Moisy1, M. Wilkinson5, C. Moureaux6, G. Wohlfahrt7,29, A. Hammerle7, L. Hörtnagl7,15, C. Gimeno8, A. Porcar-Castell9, M. Galvagno2, T. Nakaji10, J. Morison5, O. Kolle4, A. Knohl11, W. Kutsch12, P. Kolari9, E. Nikinmaa9, A. Ibrom13, B. Gielen14, W. Eugster15, M. Balzarolo14,16, D. Papale16, K. Klumpp17, B. Köstner18, T. Grünwald18, R. Joffre19, J.-M. Ourcival19, M. Hellstrom20, A. Lindroth20, C. George21, B. Longdoz22, B. Genty23,24, J. Levula9, B. Heinesch6, M. Sprintsin25, D. Yakir26, T. Manise6, D. Guyon1, H. Ahrends15,27, A. Plaza-Aguilar28, J. H. Guan4, and J. Grace3 1INRA, UMR ISPA 1391, 33140 Villenave d’Ornon, France 2Environmental Protection Agency of Aosta Valley, Climate Change Unit, ARPA Valle d’Aosta, Italy 3School of GeoSciences, University of Edinburgh, Edinburgh, EH9 3JN, UK 4Max Planck Institute for Biogeochemistry, Jena, Germany 5Forest Research, Alice Holt, Farnham, GU10 4LH, UK 6Unité de Physique des Biosystemes, Gembloux Agro-Bio Tech, Université of Liège, 5030 Gembloux, Belgium 7University of Innsbruck, Institute of Ecology, Innsbruck, Austria 8Centro de Estudios Ambientales del Mediterráneo, Paterna, Spain 9Department of Forest Sciences, University of Helsinki, P.O. Box 27, 00014, Helsinki, -

Beau Newsletter
Finding Your Niche in the Age of Digital Imaging Saturday, November 5th, 2016 Anvil Centre, New Westminster Industry Expo 10am - 5pm See the latest in cameras, lighting, printers, paper and more at the Industry Expo. Tickets to the show are included with your speaker tickets, or just come to the expo for $10 at the door. Reps from all the major brands will be on hand to demo equipment and answer your questions. Beau Newsletter - November 2016 Fusion Industry Expo • New in Used • Shooting with a Digital IR Converted Camera Canon Rebates • November 15th - It’s the Fujifilm Photo Walk with the Fuji Guys! Bag of the Month - Lowepro Pro Runner BP 350 AW • New Envy straps • Fujifilm Instax Monochrome is here! • Hasselblad H6D-100c in Rentals • more... FUSION Finding Your Niche in the Age of Digital Imaging Saturday, November 5th, 2016 2016 Anvil Centre, New Westminster, BC Use promo code Fusion 2016 encompasses many aspects of BEAU2016 to get a photography including DSLR video, travel 20% discount on the speaker series! photography, pet photography, portraits, lighting, sports photography, and much more. View the exhibition in the gallery and attend social events. Tickets are available to see individual speakers or a full day of speakers, and for the Industry Expo only. All speaker tickets include a pass to the Industry Expo. Sponsored by: Immerse yourself in photography! Attend a full day speaker series and Industry Expo. Fusion 2016 is held in conjunction with Photographie, attend two events in one! Tickets available online at - www.photographiefestival.ca www.photographiefestival.ca/fusion Industry Expo 10am - 5pm Beau Photo Supplies 1520 W. -

Evaluation of High Dynamic Range Photography As a Luminance Data Acquisition System
Lighting Res. Technol. 38,2 (2006) pp. 123Á/136 Evaluation of high dynamic range photography as a luminance data acquisition system MN Inanici PhD Lawrence Berkeley National Laboratory, Lighting Research Group, Berkeley, California, USA Received 17 January 2005; revised 21 July 2005; accepted 9 August 2005 In this paper, the potential, limitations and applicability of the High Dynamic Range (HDR) photography technique are evaluated as a luminance mapping tool. Multiple exposure photographs of static scenes were taken with a commercially available digital camera to capture the wide luminance variation within the scenes. The camera response function was computationally derived by using Photosphere software, and was used to fuse the multiple photographs into an HDR image. The vignetting effects and point spread function of the camera and lens system were determined. Laboratory and field studies showed that the pixel values in the HDR photographs correspond to the physical quantity of luminance with reasonable precision and repeatability. 1. Introduction lengthy and complex physical measurements performed in laboratory conditions, accompa- nied with extended data analysis.1,2 RGB It possible to measure the photometric proper- values were converted to the CIE tristimulus ties of a scene by means of point-by-point values through the calibration functions. measuring devices. However, these measure- These early studies were based on single ments take a long time, are prone to errors due photographs, which provide a limited dynamic to measurement uncertainties in the field and range of luminances. More recent techniques3 the obtained data may be too coarse for employ multiple photographs, which allow a analysing the lighting distribution and varia- larger luminance range to be captured.