Can Rhythm Influence Parkinson's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Krt Madonna Knight Ridder/Tribune
FOLIO LINE FOLIO LINE FOLIO LINE “Music” “Ray of Light” “Something to Remember” “Bedtime Stories” “Erotica” “The Immaculate “I’m Breathless” “Like a Prayer” “You Can Dance” (2000; Maverick) (1998; Maverick) (1995; Maverick) (1994; Maverick) (1992; Maverick) Collection”(1990; Sire) (1990; Sire) (1989; Sire) (1987; Sire) BY CHUCK MYERS Knight Ridder/Tribune Information Services hether a virgin, vamp or techno wrangler, Madon- na has mastered the public- image makeover. With a keen sense of what will sell, she reinvents herself Who’s That Girl? and transforms her music in Madonna Louise Veronica Ciccone was ways that mystify critics and generate fan interest. born on Aug. 16, 1958, in Bay City, Mich. Madonna staked her claim to the public eye by challenging social norms. Her public persona has Oh Father evolved over the years, from campy vixen to full- Madonna’s father, Sylvio, was a design en- figured screen siren to platinum bombshell to gineer for Chrysler/General Dynamics. Her leather-clad femme fatale. Crucifixes, outerwear mother, Madonna, died of breast cancer in undies and erotic fetishes punctuated her act and 1963. Her father later married Joan Gustafson, riled critics. a woman who had worked as the Ciccone Part of Madonna’s secret to success has been family housekeeper. her ability to ride the music video train to stardom. The onetime self-professed “Boy Toy” burst onto I’ve Learned My Lesson Well the pop music scene with a string of successful Madonna was an honor roll student at music video hits on MTV during the 1980s. With Adams High School in Rochester, Mich. -

Exploring Lesbian and Gay Musical Preferences and 'LGB Music' in Flanders
Observatorio (OBS*) Journal, vol.9 - nº2 (2015), 207-223 1646-5954/ERC123483/2015 207 Into the Groove - Exploring lesbian and gay musical preferences and 'LGB music' in Flanders Alexander Dhoest*, Robbe Herreman**, Marion Wasserbauer*** * PhD, Associate professor, 'Media, Policy & Culture', University of Antwerp, Sint-Jacobsstraat 2, 2000 Antwerp ([email protected]) ** PhD student, 'Media, Policy & Culture', University of Antwerp, Sint-Jacobsstraat 2, 2000 Antwerp ([email protected]) *** PhD student, 'Media, Policy & Culture', University of Antwerp, Sint-Jacobsstraat 2, 2000 Antwerp ([email protected]) Abstract The importance of music and music tastes in lesbian and gay cultures is widely documented, but empirical research on individual lesbian and gay musical preferences is rare and even fully absent in Flanders (Belgium). To explore this field, we used an online quantitative survey (N= 761) followed up by 60 in-depth interviews, asking questions about musical preferences. Both the survey and the interviews disclose strongly gender-specific patterns of musical preference, the women preferring rock and alternative genres while the men tend to prefer pop and more commercial genres. While the sexual orientation of the musician is not very relevant to most participants, they do identify certain kinds of music that are strongly associated with lesbian and/or gay culture, often based on the play with codes of masculinity and femininity. Our findings confirm the popularity of certain types of music among Flemish lesbians and gay men, for whom it constitutes a shared source of identification, as it does across many Western countries. The qualitative data, in particular, allow us to better understand how such music plays a role in constituting and supporting lesbian and gay cultures and communities. -

The Uk's Top 200 Most Requested Songs in 1980'S
The Uk’s top 200 most requested songs in 1980’s 1. Billie Jean - Michael Jackson 2. Into the Groove - Madonna 3. Super Freak Part I - Rick James 4. Beat It - Michael Jackson 5. Funkytown - Lipps Inc. 6. Sweet Dreams (Are Made of This) - Eurythmics 7. Don't You Want Me? - Human League 8. Tainted Love - Soft Cell 9. Like a Virgin - Madonna 10. Blue Monday - New Order 11. When Doves Cry - Prince 12. What I Like About You - The Romantics 13. Push It - Salt N Pepa 14. Celebration - Kool and The Gang 15. Flashdance...What a Feeling - Irene Cara 16. It's Raining Men - The Weather Girls 17. Holiday - Madonna 18. Thriller - Michael Jackson 19. Bad - Michael Jackson 20. 1999 - Prince 21. The Way You Make Me Feel - Michael Jackson 22. I'm So Excited - The Pointer Sisters 23. Electric Slide - Marcia Griffiths 24. Mony Mony - Billy Idol 25. I'm Coming Out - Diana Ross 26. Girls Just Wanna Have Fun - Cyndi Lauper 27. Take Your Time (Do It Right) - The S.O.S. Band 28. Let the Music Play - Shannon 29. Pump Up the Jam - Technotronic feat. Felly 30. Planet Rock - Afrika Bambaataa and The Soul Sonic Force 31. Jump (For My Love) - The Pointer Sisters 32. Fame (I Want To Live Forever) - Irene Cara 33. Let's Groove - Earth, Wind, and Fire 34. It Takes Two (To Make a Thing Go Right) - Rob Base and DJ EZ Rock 35. Pump Up the Volume - M/A/R/R/S 36. I Wanna Dance With Somebody (Who Loves Me) - Whitney Houston 37. -
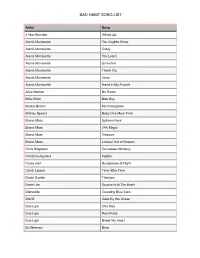
Bad Habit Song List
BAD HABIT SONG LIST Artist Song 4 Non Blondes Whats Up Alanis Morissette You Oughta Know Alanis Morissette Crazy Alanis Morissette You Learn Alanis Morissette Uninvited Alanis Morissette Thank You Alanis Morissette Ironic Alanis Morissette Hand In My Pocket Alice Merton No Roots Billie Eilish Bad Guy Bobby Brown My Prerogative Britney Spears Baby One More Time Bruno Mars Uptown Funk Bruno Mars 24K Magic Bruno Mars Treasure Bruno Mars Locked Out of Heaven Chris Stapleton Tennessee Whiskey Christina Aguilera Fighter Corey Hart Sunglasses at Night Cyndi Lauper Time After Time David Guetta Titanium Deee-Lite Groove Is In The Heart Dishwalla Counting Blue Cars DNCE Cake By the Ocean Dua Lipa One Kiss Dua Lipa New Rules Dua Lipa Break My Heart Ed Sheeran Blow BAD HABIT SONG LIST Artist Song Elle King Ex’s & Oh’s En Vogue Free Your Mind Eurythmics Sweet Dreams Fall Out Boy Beat It George Michael Faith Guns N’ Roses Sweet Child O’ Mine Hailee Steinfeld Starving Halsey Graveyard Imagine Dragons Whatever It Takes Janet Jackson Rhythm Nation Jessie J Price Tag Jet Are You Gonna Be My Girl Jewel Who Will Save Your Soul Jo Dee Messina Heads Carolina, Tails California Jonas Brothers Sucker Journey Separate Ways Justin Timberlake Can’t Stop The Feeling Justin Timberlake Say Something Katy Perry Teenage Dream Katy Perry Dark Horse Katy Perry I Kissed a Girl Kings Of Leon Sex On Fire Lady Gaga Born This Way Lady Gaga Bad Romance Lady Gaga Just Dance Lady Gaga Poker Face Lady Gaga Yoü and I Lady Gaga Telephone BAD HABIT SONG LIST Artist Song Lady Gaga Shallow Letters to Cleo Here and Now Lizzo Truth Hurts Lorde Royals Madonna Vogue Madonna Into The Groove Madonna Holiday Madonna Border Line Madonna Lucky Star Madonna Ray of Light Meghan Trainor All About That Bass Michael Jackson Dirty Diana Michael Jackson Billie Jean Michael Jackson Human Nature Michael Jackson Black Or White Michael Jackson Bad Michael Jackson Wanna Be Startin’ Something Michael Jackson P.Y.T. -

The Medic Droid Into the Groove
Into The Groove The Medic Droid And you can dance for inspiration Set me up a line, I'm waiting Get into the groove Boy you've got to prove your love to me, yeah Get up on your feet, yeah step to the beat Boy what will it be? Music can be such a revelation Dancing around, you feel the sweet sensation We might be lovers if the rhythm's right I hope this feeling never ends tonight Only when I'm dancing can I feel this free At night I lock the doors, where no one else can see I'm tired of dancing here all by myself Tonight I wanna dance with someone else Get into the groove Boy you've got to prove your love to me, yeah Get up on your feet, yeah step to the beat Boy what will it be? Gonna get to know you in a special way This doesn't happen to me every day Don't try to hide it, love wears no disguise I see the fire burning in your eyes Only when I'm dancing can I feel this free At night I lock the doors, where no one else can see I'm tired of dancing here all by myself Tonight I wanna dance with someone else Get into the groove Boy you've got to prove your love to me, yeah Get up on your feet, yeah step to the beat Boy what will it be? Live out your fantasies here with me Just let the music set you free Touch my body now and move in time Now I know you're mine, you've got to Live, live, live, live, live, live, live Live out your fantasies here with me Just let the music set you free Touch my body now and move in time Now I know you're mine Get into the groove Boy you've got to prove your love to me, yeah Get up on your feet, yeah step to the beat Boy what will it be? Get into the groove Boy you've got to prove your love to me, yeah Get up on your feet, yeah step to the beat Boy what will it be? Get into the groove Boy you've got to prove your love to me, yeah Get up on your feet, yeah step to the beat Boy what will it be? Tištěno z pisnicky-akordy.cz Sponzor: www.srovnavac.cz - vyberte si pojištění online! Powered by TCPDF (www.tcpdf.org). -

Stone Me Into the Groove Download
Stone me into the groove download Download MP3 Songs Free Online Stone me into the groove by atomic 3 MP3 youtube downloader music free download - Search for your favorite. Stone Me Into The Groove — A Car Crash In The Blue — Atomic Swing. Album cover A Car Crash In The Blue. Download Play now. Artist: Atomic Swing. Convert Youtube ATOMIC SWING / Stone Me Into The Groove to MP3 instantly. Watch the video, get the download or listen to Atomic Swing – Stone Me Into The Groove for free. Stone Me Into The Groove appears on the album A Car Crash. Download free: Atomic Swing Stone Me Into The 3. Please enter the characters you see in the image below: Similar mp3's. Atomic Swing - Stone Me. Download free: Fan Made Stone Me Into The Groove Atomic 3. Please enter the characters you see in the image below. Atomic Groove mp3. Atomic Groove @ Belly Up Tavern Solana Beach CA 6/24/mp3. Play | Download. ATOMIC SWING / Stone Me Into The 3. Deee lite - Groove is in the heart (Botteon 2k17 reWork)**Free Download**.mp3. Play | Download. ATOMIC SWING / Stone Me Into The 3. Switch browsers or download Spotify for your desktop. Stone Me Into The Groove. By Atomic Swing. • 1 song, Play on Spotify. 1. Stone Me Into The. duration: - size: MB. download. listen. embed. Atomic Swing - Stone Me Into the Groove - Playback (Grammisgalan ).mp4 mp3. Find a Atomic Swing - Stone Me Into The Groove first pressing or reissue. Complete your Atomic Swing collection. Shop Vinyl and CDs. Stone Me Into The Groove Lyrics: I woke up and the sun was dressed in blue / The Panavision colours ran out, out in my room / I wanna paint them where ever. -

Disgruntled Madonna Fans Sue Over Late Concert Starts
Disgruntled Madonna fans sue over late concert starts February 3, 2020 It’s just like a prayer — for relief. Two disgruntled local Madonna fans are suing the legendary singer for refunds to a pair of concerts in Brooklyn last year — because she showed up hours late and ruined their nights. Queens resident Antonio Velotta and New Jersey man Andrew Panos say they each shelled out around $300 to see the “Material Girl” at the BAM opera house in Fort Greene on Sept. 21 and Oct. 1, respectively, for her “Madame X” tour. Madonna was supposed to kick off both concerts at 8:30 p.m. — but didn’t get into the groove until 11:30 p.m. at the September show and 10:40 p.m. at the October gig, wrapping up around 1 a.m., the men say. The two men on Monday filed a class-action suit in Brooklyn Supreme Court against the “Like a Virgin” crooner, along with concert promoter Live Nation, claiming false advertising and breach of contract. “She totally screwed her fans over,” fumed Velotta, 40, who says he didn’t get home from the show until 3 a.m. The hairstylist — who says he moved to the Big Apple from Cleveland 14 years ago after seeing Madonna in the 1985 film “Desperately Seeking Susan” — at least enjoyed the performance once it got underway. But Panos, 35, told The Post he and his wife “immediately hated” the show and left early. The couple still had to pay their baby-sitter extra to watch their two kids due to the late night — they didn’t get back home to Union County, NJ, until 3 a.m. -

Madonna E Seu Acervo Multiartístico: Os Fetichismos Visuais Na Obra Do Maior Mito
Intercom – Sociedade Brasileira de Estudos Interdisciplinares da Comunicação 41º Congresso Brasileiro de Ciências da Comunicação – Joinville - SC – 2 a 8/09/2018 Madonna e seu Acervo Multiartístico: os fetichismos visuais na obra do maior mito 1 pop do século XX Rafael Nacif de Toledo PIZA2 Universidade do Estado do Rio de Janeiro - UERJ Resumo O presente artigo pretende fazer uma retrospectiva da carreira da cantora Madonna, tendo como ponto de tensionamento os fetichismos visuais, conceito desenvolvido pelo italiano Massimo Canevacci em 2008. Nosso intuito é analisar o desenvolvimento cronológico da obra da cantora, relacionando suas iniciativas ao escopo teórico criado pelo sociólogo italiano, oferecendo pistas para decifrarmos os segredos de sua longevidade e do sucesso por trás de sua persona pública. Palavras-chave Madonna, cronologia, fetichismos visuais. Introdução Ser fã de Madonna é um privilégio e uma honra. Acompanhar a carreira de uma cantora, atriz, produtora, diretora de cinema, celebridade e personalidade é uma tarefa das mais prazerosas e dispendiosas. Montar uma coleção de objetos da Madonna pode custar alguns bons reais que poderiam ser investidos em outras coisas, como carros, roupas e outros itens de consumo. Mas deixando o lado mercantil de lado e focando na parte estética, o que Madonna nos apresenta é uma coleção de hits para as pistas de dança e baladas empacotados em formatos correspondentes às tecnologias do período, sempre com fotografias riquíssimas e figurinos ousados, tipologias inovadoras e designs de cair o queixo. Madonna e os fetichismos visuais de Canevacci Em 2008, Massimo Canevacci desenvolve “Fetichismos visuais: corpos erópticos e metrópole comunucacional” (Ateliê Editorial, 2008). -

Into the Groove
Stephen Bray/Madonna 80's Pop 1985 BPM: 116 Aflyttet af Martin Krøger 2002 Into The Groove Madonna – Desperately Seeking Susan (1985) Intro And you can dance – for inspiration ||: Cm | Abmaj :|| Come on – I'm waiting ||: Cm | Abmaj :|| B1 Get into the groove – boy you've got to prove | Cm | Abmaj | Your love to me, yeah | Cm | Abmaj – Bb | Get up on your feet, yeah – step to the beat | Cm | Abmaj | Boy what will it be | Cm | Abmaj – Bb | A1 Music can be such a revelation | Cm – Gm7 | Fm7 – Gm7 | Dancing around you feel the sweet sensation | Cm – Gm7 | Fm7 – Gm7 | We might be lovers if the rhythm's right | Cm – Gm7 | Fm7 – Gm7 | I hope this feeling never ends tonight | Cm – Gm7 | Fm7 – Gm7 | Only when I'm dancing can I feel this free | Cm – Gm7 | Fm7 – Gm7 | At night I lock the doors, where no one else can see | Cm – Gm7 | Fm7 – Gm7 | I'm tired of dancing here all by myself | Cm – Gm7 | Fm7 – Gm7 | Tonight I wanna dance with someone else | Cm – Gm7 | Fm7 – Gm7 | B2 Get into the groove – boy you've got to prove | Cm | Abmaj | Your love to me, yeah | Cm | Abmaj – Bb | Get up on your feet, yeah – step to the beat | Cm | Abmaj | Boy what will it be | Cm | Abmaj – Bb | A2 Gonna get to know you in a special way | Cm – Gm7 | Fm7 – Gm7 | This doesn't happen to me every day | Cm – Gm7 | Fm7 – Gm7 | Don't try to hide it love wears no disguise | Cm – Gm7 | Fm7 – Gm7 | I see the fire burning in your eyes | Cm – Gm7 | Fm7 – Gm7 | Only when I'm dancing can I feel this free | Cm – Gm7 | Fm7 – Gm7 | At night I lock the doors, where no one else can see -

Cryptic Match-Ups: Madonna Mindfood Wheel Words
Match-Ups: Madonna Cryptic Work out all the missing parts from these Madonna songs and find the Use the cryptic clues below to solve this crossword with a twist. words hidden in the box of letters. The letters left over will spell out a film Madonna starred in that featured one of the songs. I N S E C T S S W E P T U P N P A T E R S T B D E S Y F I T S U J A T E E L E A D E N C A S E H A O H C A E R P P Y E N I E L M S R V U G R G N T A K E L B A N Y M A D E T O M E A S U R E D I F L A I R E T A M A A M A O E W N L O L L E T I T U B Y R N M O T I P S T E R S H O I S T E H F S U E U T E N A E G E O U R A I L E D P A R T T I M E C S F F L Y S E R B T H E A N B C C H A K R E E I S N T O M H R T T F E A T H E R W E I G H T Y U Y K E T E E U E E E R H L R O O M R B Z O K K I L U I N M R Y A E R A S B R U N E I A C E A Y N N N L S A D E G A E N M E Y L C L R S A G R O O V E R U R H S T E P P E S M I L D E S T C S P E M I T D E B A P N ACROSS 28. -

Blonde Ambition Song List
Blonde Ambition Song List 1, 2, 3................................................................... Gloria Estefan All Summer Long ................................................... Kid Rock Ain’t No Other Man ................................................ Christina Aguilera At Last .................................................................. Etta James Baby Got Back ....................................................... Sir Mix A Lot Bad....................................................................... Michael Jackson Bailamos ............................................................... Enrique Iglesias Barracuda.............................................................. Heart Beat It .................................................................. Michael Jackson Believe .................................................................. Cher Before He Cheats ................................................... Carrie Underwood Billie Jean.............................................................. Michael Jackson Black Cat ............................................................... Janet Jackson Black Horse And A Cherry Tree ................................ Kt Tunstall Black Velvet .......................................................... Alannah Myles Bleeding Love ........................................................ Leona Lewis Blue Suede Shoes .................................................. Elvis Boogie Oogie ......................................................... A Taste Of Honey Brick House -

Legends Patchlist
Legends Patchlist 1984 Rez Bass Head Over Heels Brass Pretty Young Bass 1984 Strings Here Comes The Rain Again Radio Ga Ga Arps 99 Balloons Bass Holiday Bass Radio Ga Ga Strings 99 Balloons Strings Holiday Keys Ran So Far Away Strings Axel F Bass Holiday Lead Rebel Yell Seq Axel F Keys Holiday Strings Rebel Yell Lead Axel F Lead I Don’t Care Anymore Rebel Yell Strings Baby Be Mine Funky Lead I Wanna Be Your Lover Rio Arps Baby Be Mine Soft Lead In the Air Tonight Rock With You Beautiful Ones Into The Groove Bass Separate Ways Harpsichord Break Free Solo Into The Groove Keys Sisters Doin’ It For Chemistry Pad Invisible Sun Themselves Comp Blue Stabs It’s a Sin Lead Sweet Dreams Lead Controversy Strings Jump (For My Love) Brass Sweet Dreams String 1 Countdown Solo Jump (For My Love) Hi Sweet Dreams String 2 Countdown Strings String Subdivisions Brass Doctor Doctor Sequence Just What I Needed Tainted Love Bass Doctor Doctor Lead Kids in America Bass Take On Me Lead Doctor Doctor 5th Lead Kids in America Perc The Camera Eye Brass Doves Cry Keys Kids in America Strings The Camera Eye Pad Doves Cry Strings La Isla Bonita Bass The Final Countdown Dress You Up Bass Let’s Go Crazy Organ The Way You Make Bass Dress You Up Strings Let’s Go Lead The Way You Make Brass Dress You Up Xylophone Let’s Go String Thriller Bass Drive Lead Like a Virgin Bass Tom Sawyer Lead Drive Pad 1 Like It’s 1999 Tom Sawyer Rez Bass Drive Pad 2 Losing It Arps Waiting for a Girl Keys Every Little Thing She Does Might As Well Jump Waiting for a Girl Lead Eyes Without a Face New Song Bass Wanna Be Startin’ Bass Fade to Grey Bass New Song Percussion West End Bass Fade to Grey Bell New Song Solo When U Were Mine Fade to Grey Strings Nothing Compares 2 U Why U Wanna Organ Gimme Gimme Bass Numan String You Got Lucky Brass Gimme Gimme Lead O My God Pad You Got Lucky Flute.