The Structure and Calcification of the Crustacean Cuticle1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Can You Engineer an Insect Exoskeleton?
Page 1 of 14 Can you Engineer an Insect Exoskeleton? Submitted by Catherine Dana and Christina Silliman EnLiST Entomology Curriculum Developers Department of Entomology, University of Illinois Grade level targeted: 4th Grade, but can be easily adapted for later grades Big ideas: Insect exoskeleton, engineering, and biomimicry Main objective: Students will be able to design a functional model of an insect exoskeleton which meets specific physical requirements based on exoskeleton biomechanics Lesson Summary We humans have skin and bones to protect us and to help us stand upright. Insects don’t have bones or skin but they are protected from germs, physical harm, and can hold their body up on their six legs. This is all because of their hard outer shell, also known as their exoskeleton. This hard layer does more than just protect insects from being squished, and students will get to explore some of the many ways the exoskeleton protects insects by building one themselves! For this fourth grade lesson, students will work together in teams to use what they learn about exoskeleton biomechanics to design and build a protective casing. To complete the engineering design cycle, students can use what they learn from testing their case to redesign and re-build their prototype. Prerequisites No prior knowledge is required for this lesson. Students should be introduced to the general form of an insect to facilitate identification of the exoskeleton. Live insects would work best for this, but pictures and diagrams will work as well. Instruction Time 45 – 60 minutes Next Generation Science Standards (NGSS) Framework Alignment Disciplinary Core Ideas LS1.A: Structure and Function o Plants and animals have both internal and external structures that serve various functions in growth, survival, behavior, and reproduction. -

Do You Think Animals Have Skeletons Like Ours?
Animal Skeletons Do you think animals have skeletons like ours? Are there any bones which might be similar? Vertebrate or Invertebrate § Look at the words above… § What do you think the difference is? § Hint: Break the words up (Vertebrae) Vertebrates and Invertebrates The difference between vertebrates and invertebrates is simple! Vertebrates have a backbone (spine)… …and invertebrates don’t Backbone (spine) vertebrate invertebrate So, if the animal has a backbone or a ‘vertebral column’ it is a ‘Vertebrate’ and if it doesn’t, it is called an ‘Invertebrate.’ It’s Quiz Time!! Put this PowerPoint onto full slideshow before starting. You will be shown a series of animals, click if you think it is a ‘Vertebrate’ or an ‘Invertebrate.’ Dog VertebrateVertebrate or InvertebrateInvertebrate Worm VertebrateVertebrate or InvertebrateInvertebrate Dinosaur VertebrateVertebrate or InvertebrateInvertebrate Human VertebrateVertebrate or InvertebrateInvertebrate Fish VertebrateVertebrate or InvertebrateInvertebrate Jellyfish VertebrateVertebrate or InvertebrateInvertebrate Butterfly VertebrateVertebrate or InvertebrateInvertebrate Types of Skeleton § Now we know the difference between ‘Vertebrate’ and ‘Invertebrate.’ § Let’s dive a little deeper… A further classification of skeletons comes from if an animal has a skeleton and where it is. All vertebrates have an endoskeleton. However invertebrates can be divided again between those with an exoskeleton and those with a hydrostatic skeleton. vertebrate invertebrate endoskeleton exoskeleton hydrostatic skeleton What do you think the words endoskeleton, exoskeleton and hydrostatic skeleton mean? Endoskeletons Animals with endoskeletons have Endoskeletons are lighter skeletons on the inside than exoskeletons. of their bodies. As the animal grows so does their skeleton. Exoskeletons Animals with exoskeletons Watch the following have clip to see how they shed their skeletons on their skeletons the outside! (clip the crab below). -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
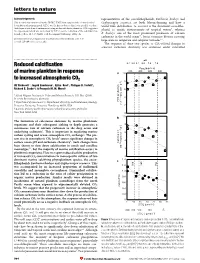
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Penile Circular Fasciocutaneous Flaps for Complex Anterior Urethral Strictures K.J
18 Penile Circular Fasciocutaneous Flaps for Complex Anterior Urethral Strictures K.J. Carney, J.W. McAninch 18.1 Penile Fascial Anatomy – 146 18.2 Flap Anatomy – 148 18.3 Patient Selection – 148 18.4 Preoperative Preparation – 148 18.5 Patient Positioning – 148 18.6 Flap Harvest – 149 18.7 Stricture Exposure – 150 18.8 Anastomosis – 151 18.9 Postoperative Care – 152 References – 152 146 Chapter 18 · Penile Circular Fasciocutaneous Flaps for Complex Anterior Urethral Strictures Surgical reconstruction of complex anterior urethral stric- Buck’s fascia is a well-defined fascial layer that is close- tures, 2.5–6 cm long, frequently requires tissue-transfer ly adherent to the tunica albuginea. Despite this intimate techniques [1–8]. The most successful are full-thickness association, a definite plane of cleavage exists between the free grafts (genital skin, bladder mucosa, or buccal muco- two, permitting separation and mobilization. Buck’s fascia sa) or pedicle-based flaps that carry a skin island. Of acts as the supporting layer, providing the foundation the latter, the penile circular fasciocutaneous flap, first for the circular fasciocutaneous penile flap. Dorsally, the described by McAninch in 1993 [9], produces excel- deep dorsal vein, dorsal arteries, and dorsal nerves lie in a lent cosmetic and functional results [10]. It is ideal for groove just deep to the superficial lamina of Buck’s fascia. reconstruction of the distal (pendulous) urethra, where The circumflex vessels branch from the dorsal vasculature the decreased substance of the corpus spongiosum may and lie just deep to Buck’s fascia over the lateral aspect jeopardize graft viability. -

Distribution of Calcium Phosphate in the Exoskeleton of Larval Exeretonevra Angustifrons Hardy (Diptera: Xylophagidae)
Arthropod Structure & Development 34 (2005) 41–48 www.elsevier.com/locate/asd Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae) Bronwen W. Cribba,b,*, Ron Rascha, John Barrya, Christopher M. Palmerb,1 aCentre for Microscopy and Microanalysis, The University of Queensland, Brisbane, Qd 4072, Australia bDepartment of Zoology and Entomology, The University of Queensland, Brisbane, Qd 4072, Australia Received 28 July 2004; accepted 26 August 2004 Abstract Distribution and organisation of the mineral, amorphous calcium phosphate (ACP), has been investigated in the exoskeleton of the xylophagid fly larva Exeretonevra angustifrons Hardy. While head capsule and anal plate are smooth with a thin epicuticle, the epicuticle of the body is thicker and shows unusual micro-architecture comprised of minute hemispherical (dome-shaped) protrusions. Electron microprobe analysis and energy dispersive spectroscopy revealed heterogeneity of mineral elements across body cuticle and a concentration of ACP in the epicuticle, especially associated with the hemispherical structures. Further imaging and analysis showed the bulk of the ACP to be present in nano-sized granules. It is hypothesised that the specific distribution of ACP may enhance cuticular hardness or durability without reducing flexibility. q 2004 Elsevier Ltd. All rights reserved. Keywords: Insect; Cuticle; Integument; Hardening; Analytical electron microscopy; Electron microprobe 1. Introduction was distributed heterogeneously. Further investigation of the distribution of the mineral phase at the micron and Strengthening of biological structures through cuticular nanometre level is needed to discover where deposition is calcification is well developed in decapod crustaceans but it occurring and how this might affect exoskeletal organis- rarely occurs in insects, where it is poorly understood ation. -

Effects of CO2-Induced Ph Reduction on the Exoskeleton Structure and Biophotonic Properties of the Shrimp Lysmata Californica
UC San Diego UC San Diego Previously Published Works Title Effects of CO2-induced pH reduction on the exoskeleton structure and biophotonic properties of the shrimp Lysmata californica. Permalink https://escholarship.org/uc/item/3mj6d2n4 Journal Scientific reports, 5(1) ISSN 2045-2322 Authors Taylor, Jennifer RA Gilleard, Jasmine M Allen, Michael C et al. Publication Date 2015-06-01 DOI 10.1038/srep10608 Peer reviewed eScholarship.org Powered by the California Digital Library University of California www.nature.com/scientificreports OPEN Effects of CO2-induced pH reduction on the exoskeleton structure and biophotonic Received: 10 November 2014 Accepted: 07 April 2015 properties of the shrimp Lysmata Published: 01 June 2015 californica Jennifer R. A. Taylor1, Jasmine M. Gilleard2, Michael C. Allen1 & Dimitri D. Deheyn1 The anticipated effects of CO2-induced ocean acidification on marine calcifiers are generally negative, and include dissolution of calcified elements and reduced calcification rates. Such negative effects are not typical of crustaceans for which comparatively little ocean acidification research has been conducted. Crustaceans, however, depend on their calcified exoskeleton for many critical functions. Here, we conducted a short-term study on a common caridean shrimp, Lysmata californica, to determine the effect of CO2-driven reduction in seawater pH on exoskeleton growth, structure, and mineralization and animal cryptic coloration. Shrimp exposed to ambient (7.99 ± 0.04) and reduced pH (7.53 ± 0.06) for 21 days showed no differences in exoskeleton growth (percent increase in carapace length), but the calcium weight percent of their cuticle increased significantly in reduced pH conditions, resulting in a greater Ca:Mg ratio. -

Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs
Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs Coral reefs are vital to the long-term to produce CaCO3, carbon dioxide (CO2), As CO2 increases in the atmosphere, viability of coastal societies, providing and water (H2O). Over time as these more is absorbed by the surface of the economic, recreational, and aesthetic organisms grow and die, their skeletons ocean, where it combines with seawater value from which coastal communities break down and become calcium carbon- to make a weak acid called carbonic thrive. Some of the services that coral ate sediments. These sediments fill in acid (H2CO3). This process, called reefs provide include protection from the framework of the reef and eventually ocean acidification, causes a decrease storm waves, nurseries and habitats for become cemented together, construct- in seawater pH (or increase in acidity) commercially important fish species, ing the foundation for continued upward that can result in a decrease in biogenic and production of sand for beaches. growth of the reef structure. The infilling calcification rates, dissolution of carbon- Coral reefs develop over thousands of the reef framework with sediments is ate sediments, and loss of reef structure. of years as tropical marine organ- what allows vertical accretion over time One of the primary concerns associated isms build skeletons of calcium carbonate and enables reef growth to keep up with with ocean acidification is whether coral (CaCO3) minerals to form a three-dimen- sea-level rise. Calcification is a revers- reefs will be able to continue to grow at sional structure (fig. 1). This process, ible process. -

Effect of Ph Change on Exoskeletons of Selected Saltwater Organisms Which Rely on Calcium Fixation Derya Z
Journal of Emerging Investigators Effect of pH change on exoskeletons of selected saltwater organisms which rely on calcium fixation Derya Z. Tansel1, Ariadna Arreaza2, Berrin Tansel2 1 Coral Gables Senior High, Coral Gables, FL 2 Florida International University, Miami, FL Summary increase in H+ concentration in the last 200 years (1,2,3). The projections for rising atmospheric carbon dioxide According to atmospheric CO2 projections, ocean surface concentrations indicate that the pH levels of the ocean pH levels are estimated to decrease by 0.3-0.4 units by surface could decrease by 0.3-0.4 units by the end of the end of the 21st century. This decrease corresponds the 21st century. The objective of this research was to to an increase in the hydrogen ion concentration of about evaluate the effect of pH on the exoskeletons of six aquatic organisms commonly found in South Florida 100-150% above the levels in the late 1800s (4,5). The coastal waters. The exoskeleton samples studied were impacts of ocean acidification can be 10–50% higher from the common nutmeg (Cancellaria reticulate), near coastal areas due to proximity to anthropogenic lettered olive (Oliva sayana), stiff pen shell (Atrina rigida), sources (6). kitten’s paw (Plicatulidae), fan coral (Gorgonia ventalina), Although some species can tolerate pH changes, and common slipper shell (Crepidula fornicate). The many marine organisms and processes can be impacted, exoskeleton samples were exposed to saltwater (34% including the composition of communities and food webs salinity) at pH levels ranging from 8.3 to 6.0 for 5 days. -

Local Seashells Provide More Than Just a Home for Hermit Crabs by Jennifer Peura
Refuge Notebook • Vol. 17, No. 45 • November 6, 2015 Local seashells provide more than just a home for hermit crabs by Jennifer Peura Gastropod shells collected near St. Lawrence Island were studied to assess if their populations affected hermit crab populations. After beachcombing at low tide, perchance coin- of calcium carbonate, the major compound in shells, ciding with a full or new moon to maximize your suc- coral, the exoskeleton of lobsters and crabs, and (im- cess, be it at Captain Cook State Park or Homer’s portantly to Sockeye lovers) pteropods. Pteropods are Bishop’s Beach, you’re likely to end up with a small the major food source of krill, which in turn is the ma- collection of shells, crab exoskeletons, or other rem- jor food source for juvenile salmon. Sea shells also nants of marine life. Even without regard to the ac- provide habitats for multiple fish species at different tual animals whose exoskeleton remains are now sim- stages in their life cycle, notably to hide from preda- ply just seashells that adorn the mantle in your living tion. Shells are used by shorebirds to build nests, by room, the shells themselves have other ecological val- barnacles as a substrate to grow on, and by hermit ues. crabs for protection. The breakdown of shells provides nutrients for or- The lowly hermit crab you might purchase from ganisms that live in the ocean’s benthos (or bottom). PetCo is often assumed to be a textbook example of This nutrient recycling is integral to the availability one species that can’t exist without another in close 86 USFWS Kenai National Wildlife Refuge Refuge Notebook • Vol. -

The Biophysical Modeling of the Human Tegument
International Research Journal of Pharmacy and Medical Sciences ISSN (Online): 2581-3277 The Biophysical Modeling of the Human Tegument Janos Vincze, Gabriella Vincze-Tiszay Health Human International Environment Foundation, Budapest, Hungary Email address: [email protected] Abstract— Sensory organs are parts of our body, which collect and transmit information to the central nervous system about the outside world, and about the internal condition of our body. The body collects information by millions of microscopic structures, the so-called receptor cells. These can be found in almost all parts of the body, skin, muscle, joints, internal organs, in the walls of blood-vessels and in specialized organs such as the eye or the inner ear. There are numerous types of receptor cells in the skin. Some of them register mechanical stress or pressure, others the position and displacement of sensory hairs. Different stimulation cause different sensations, such as pain, tickling, hard or light pressure, heat or cold. The nerve fibres connected to the receptor transform stimuli above threshold into sequence of actions potentials. We modelling the action potential in the mathematical equation. Although our current knowledge regarding the mechanisms of pain development is incomplete, a number of pain-related phenomena have been discovered, which bear importance from both pathobiophysical and clinical aspects. Skin is an important receptive field, due to the numerous and various terminations of the cutaneous analyser which informs the nervous centres on the proprieties and phenomena that the body gets in contact with. Keywords— human tegument, hand nail, action potential, pain reception. loosing the function of one or more analysers, reach a special I.