Chapter 8 – State of the Bay, Third Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
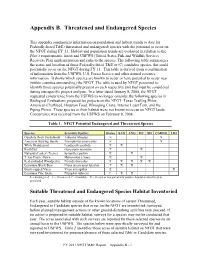
Appendix B. Threatened and Endangered Species
Appendix B. Threatened and Endangered Species This appendix summarizes information on population and habitat trends to date for Federally-listed T&E (threatened and endangered) species with the potential to occur on the NFGT during FY 11. Habitat and population trends are evaluated in relation to the Plan’s requirements, forest and USFWS (United States Fish and Wildlife Service) Recovery Plan implementation and risks to the species. The following table summarizes the status and location of those Federally-listed T&E or (C) candidate species that could potentially occur on the NFGT during FY 11. This table is derived from a combination of information from the USFWS, U.S. Forest Service and other natural resource information. It shows which species are known to occur or have potential to occur near (within counties surrounding) the NFGT. The table is used by NFGT personnel to identify those species potentially present on each respective unit that must be considered during site-specific project analysis. In a letter dated January 8, 2008, the NFGT requested concurrence from the USFWS to no longer consider the following species in Biological Evaluations prepared for projects on the NFGT: Texas Trailing Phlox, American Chaffseed, Houston Toad, Whooping Crane, Interior Least Tern, and the Piping Plover. These species or their habitat were not known to occur on NFGT lands. Concurrence was received from the USFWS on February 8, 2008. Table 1. NFGT Potential Endangered and Threatened Species Species Scientific Epithet Status SAB ANG DC SH CADDO -

Texas Prairie Dawn-Flower (Hymenoxys Texana) 5-Year Review
Texas prairie dawn-flower (Hymenoxys texana) 5-Year Review: Summary and Evaluation Photo credit: USFWS U.S. Fish and Wildlife Service Texas Coastal Ecological Services Field Office Houston, Texas Table of Contents ABBREVIATIONS ........................................................................................................................ 3 1.0 GENERAL INFORMATION .......................................................................................... 4 2.0 REVIEW ANALYSIS ...................................................................................................... 7 2.4 SYNTHESIS .................................................................................................................. 24 3.0 RESULTS....................................................................................................................... 25 4.0 RECOMMENDATIONS FOR FUTURE ACTIONS.................................................... 26 5.0 REFERENCES ............................................................................................................... 28 Appendix A ................................................................................................................................... 31 Recommendation resulting from the 5-Year Review: .................................................................. 34 Figures Figure 1 Current H. texana county occurrences ............................................................................. 9 Tables Table 1 Renaming of species historically associated with H. texana .......................................... -

PSD GHG Biological Assessment for Air Liquide
Biological Assessment Report Air Liquide Bayou Cogeneration Plant Pasadena, Texas June 18, 2013 www.erm.com Delivering sustainable solutions in a more competitive world Air Liquide Biological Assessment Report June 18, 2013 Project No. 0151579 Bayou Cogeneration Plant Pasadena, Texas Peter T. Belmonte, P.E. Partner-in-Charge Siddharth (Sid) Rajmohan Project Manager Environmental Resources Management 15810 Park Ten Place, Suite 300 Houston, Texas 77084-5140 T: 281-600-1000 F: 281-600-1001 Texas Registered Engineering Firm F-2393 TABLE OF CONTENTS EXECUTIVE SUMMARY VI 1.0 INTRODUCTION 1 1.1 SITE LOCATION AND HISTORY 1 1.2 PROJECT PURPOSE AND NEED 2 2.0 THREATENED AND ENDANGERED SPECIES REGULATORY FRAMEWORK4 2.1 FEDERAL REGULATIONS 5 2.1.1 Federal Endangered Species Act 5 3.0 IDENTIFICATION OF ACTION AREA 6 4.0 PROJECT DESCRIPTION 9 4.1 PROJECT LOCATION 9 4.2 EMISSIONS CONTROLS 9 4.3 NOISE LEVELS 11 4.4 DUST 11 4.5 WASTEWATER 11 5.0 ENVIRONMENTAL BASELINE 12 5.1 METHODOLOGY 12 5.2 FEDERALLY LISTED THREATENED AND ENDANGERED SPECIES, DESIGNATED CRITICAL HABITAT, AND OTHER PROTECTED SPECIES OF POTENTIAL OCCURRENCE IN THE ACTION AREA 12 5.3 FEDERALLY LISTED THREATENED AND ENDANGERED SPECIES AND DESIGNATED CRITICAL HABITAT 13 5.3.1 Texas Prairie dawn-flower (Federal Endangered) 13 5.3.2 West Indian manatee (Federal Endangered) 14 5.3.3 Houston Toad 15 5.3.4 Red-Cockaded Woodpecker 15 5.3.5 Whooping Crane 16 5.3.6 Smalltooth Sawfish 17 5.3.7 Louisiana Black Bear 17 5.3.8 Red Wolf 18 5.3.9 Green Sea Turtle 18 5.3.10 Kemp’s Ridley Sea Turtle 19 -

Hymenoxys Texana Mitigation for West Greens Road Harris County, Texas
Hymenoxys texana Mitigation for West Greens Road Harris County, Texas Endangered Plant Protected by the Endangered Species Act CEER July 30, 2014 2 Discussion • Background • Implementation • Habitat Review • Long-term Protection • Mitigation Plan 3 The Plant 100 A 90 B C 80 D 70 E 60 F 50 G Axis Label Axis - H Y 40 30 20 10 0 A B C D E F G H X-Axis Label 4 How Plants are Protected • Endangered and threatened plant species on public lands are fully protected under ESA • On private lands they are not fully protected • To take, possess, transport, or sell protected plants for commercial purposes, a permit is required • On private lands, the plant, habitat, etc. can be removed and/or destroyed without punishment 5 Background on West Greens Road Segment A • Houston has one of the fastest growing populations in the United States • Additional infrastructure, including roads, is needed • Harris County Public Infrastructure Department (HCPID) wanted to use federal funds for construction of West Greens Road extension • Use of federal funds required following the National Environmental Policy Act, etc. • Environmental Assessment was started in 2001 6 Environment Assessment • Requires review of threatened and endangered species • Harris County, Texas has only one Federally protected plant species Hymenoxys texana (Coulter & Rose) Cockrell • In 1998, a review of the proposed right-of-way (ROW) indicated potential H. texana habitat • H. texana was confirmed to be present in 2003 7 Need for a Biological Assessment • When the land is acquired by HCPID, the plant and habitat is protected • Taking of any T&E species requires consultation with U.S. -

The Ecology and Sociology of the Mission-Aransas Estuary
THE ECOLOGY AND SOCIOLOGY OF THE MISSION-ARANSAS ESTUARY THE MISSION-ARANSAS ESTUARY OF AND SOCIOLOGY THE ECOLOGY THE ECOLOGY AND SOCIOLOGY OF THE MISSION-ARANSAS ESTUARY AN ESTUARINE AND WATERSHED PROFILE Evans, Madden, and Morehead Palmer Edited by Anne Evans, Kiersten Madden, Sally Morehead Palmer THE ECOLOGY AND SOCIOLOGY OF THE MISSION-ARANSAS ESTUARY AN ESTUARINE AND WATERSHED PROFILE Edited by Anne Evans, Kiersten Madden, Sally Morehead Palmer On the cover: Photo courtesy of Tosh Brown, www.toshbrown.com TABLE OF CONTENTS Acknowledgments………………………………………………………………………………………………......ix Contributors…………………………………………………………………………………………………….…....xi Executive Summary………………………………………………………………………………………………..xiii Chapter 1 Introduction ........................................................................................ 1 Reserve Mission, Vision, and Goals ...................................................................... 2 Chapter 2 Biogeographic Region ....................................................................... 5 Chapter 3 Physical Aspects ................................................................................ 7 References ........................................................................................................... 10 Chapter 4 Climate .............................................................................................. 13 Issues of Concern for Climate .............................................................................. 14 Climate Change ............................................................................................ -

FEIS Citation Retrieval System Keywords
FEIS Citation Retrieval System Keywords 29,958 entries as KEYWORD (PARENT) Descriptive phrase AB (CANADA) Alberta ABEESC (PLANTS) Abelmoschus esculentus, okra ABEGRA (PLANTS) Abelia × grandiflora [chinensis × uniflora], glossy abelia ABERT'S SQUIRREL (MAMMALS) Sciurus alberti ABERT'S TOWHEE (BIRDS) Pipilo aberti ABIABI (BRYOPHYTES) Abietinella abietina, abietinella moss ABIALB (PLANTS) Abies alba, European silver fir ABIAMA (PLANTS) Abies amabilis, Pacific silver fir ABIBAL (PLANTS) Abies balsamea, balsam fir ABIBIF (PLANTS) Abies bifolia, subalpine fir ABIBRA (PLANTS) Abies bracteata, bristlecone fir ABICON (PLANTS) Abies concolor, white fir ABICONC (ABICON) Abies concolor var. concolor, white fir ABICONL (ABICON) Abies concolor var. lowiana, Rocky Mountain white fir ABIDUR (PLANTS) Abies durangensis, Coahuila fir ABIES SPP. (PLANTS) firs ABIETINELLA SPP. (BRYOPHYTES) Abietinella spp., mosses ABIFIR (PLANTS) Abies firma, Japanese fir ABIFRA (PLANTS) Abies fraseri, Fraser fir ABIGRA (PLANTS) Abies grandis, grand fir ABIHOL (PLANTS) Abies holophylla, Manchurian fir ABIHOM (PLANTS) Abies homolepis, Nikko fir ABILAS (PLANTS) Abies lasiocarpa, subalpine fir ABILASA (ABILAS) Abies lasiocarpa var. arizonica, corkbark fir ABILASB (ABILAS) Abies lasiocarpa var. bifolia, subalpine fir ABILASL (ABILAS) Abies lasiocarpa var. lasiocarpa, subalpine fir ABILOW (PLANTS) Abies lowiana, Rocky Mountain white fir ABIMAG (PLANTS) Abies magnifica, California red fir ABIMAGM (ABIMAG) Abies magnifica var. magnifica, California red fir ABIMAGS (ABIMAG) Abies -

FINAL USFWS Biological Opinion Dallas to Houston High Speed Rail
United States Department of the Interior FISH AND WILDLIFE SERVICE Division of Ecological Services 17629 El Camino Real, Suite 211 In Reply Refer To: 281/286-8282 / (FAX) 281/488-5882 FWS/R2/02ETT X00-2019-F- 2135 July 8, 2020 Michael Johnsen U.S. Department of Transportation Federal Railroad Administration Attention: Kevin Wright 1200 New Jersey Avenue, SE., MS-20 Washington D.C., 20590 Subject: Biological Opinion for the Dallas to Houston High-Speed Rail; Dallas, Ellis, Navarro, Freestone, Limestone, Leon, Madison, Grimes, Waller, and Harris counties, Texas Dear Mr. Johnsen: This document transmits the U.S. Fish and Wildlife Service’s (Service) Biological Opinion based on our review of the effects of the proposed Dallas to Houston High-Speed Rail Project (Project) on the federally endangered large-fruited sand-verbena (Abronia macrocarpa) (LFSV) and the federally endangered Navasota ladies’-tresses (Spiranthes parksii) (NLT) pursuant to section 7(a)(2) of the Endangered Species Act of 1973 (Act), as amended (16 U.S.C. 1531 et seq.). There is no critical habitat for either the LFSV or NLT within the Project’s Action Area. We originally received a request for formal consultation on December 13, 2019. However in order to fulfill the formal Section 7 process as outlined under the Act, we requested additional species and project-specific information in order to complete our Biological Opinion. We received a revised Biological Assessment on April 28, 2020. Although we received an updated request for formal consultation on January 9, 2020, upon agreeance with the federal action agency, Federal Railroad Administration (FRA), we jointly agreed to provide a final Biological Opinion on or before July 1, 2020. -

Grand Parkway State Highway 99 Segment E
Final Environmental VOLUME Impact Statement II From IH 10 to US 290 Grand Parkway Harris County, Texas State Highway 99 Segment E FHWA-TX-EIS-02-01-F November 2007 Document Organization The Grand Parkway Segments E, F-1, F-2, and G study area is divided into four Segments of Independent Utility (SIUs) to facilitate planning, design, and construction because limited state and federal funding provides no assurance that all the Grand Parkway segments would be constructed. Each SIU would connect at least two existing major transportation corridors to ensure independent utility as well as independent significance as required by the U.S. Department of Transportation (USDOT), Federal Highway Administration (FHWA) regulations (23 Code of Federal Regulations [CFR] 771.111[f]). The Segment E Final Environmental Impact Statement (FEIS) is organized in a four-volume set in response to public and resource agency comments concerning potential cumulative effects of Segment E and the need to assess this segment as a facility with independent utility and logical termini, as follows: Volume I presents analyses and results common to all four segments, including project description, the need for and purpose of the project, alternatives considered, affected environment, indirect and cumulative effects, and agency and public coordination; Volume II provides a detailed assessment of Segment E relative to its project area. It includes issues, permits, and federal actions particular to Segment E, such as the need for and purpose of the project; alternatives considered; affected environment; environmental consequences; avoidance, minimization, and mitigation measures; indirect and cumulative effects; and agency and public coordination; Volume III contains Exhibits and Appendices referenced in Volumes I and II; and Volume IV includes materials related to the Segment E Draft Environmental Impact Statement (DEIS) Public Hearing, comments submitted, and responses provided following the release of the Segment E DEIS in February 2003. -

Literature Cited Robert W. Kiger, Editor This Is a Consolidated List Of
RWKiger 5 Jul 18 Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volumes 19, 20, and 21, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbreviated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu and E. A. Mennega (1992+). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix "a"; thus, the sequence of explicit suffixes begins with "b". Works missing from any suffixed sequence here are ones cited elsewhere in the Flora that are not pertinent in these volumes. -

The Ecology and Abundance of Hymenoxys Texana (Asteraceae)
Singhurst, JR, N. Shackelford, W. Newman, J.N. Mink, and W.C. Holmes. 2014. The ecology and abundance of Hymenoxys texana (Asteraceae). Phytoneuron 2014-19: 1–19. Published 21 January 2014. ISSN 2153 733X THE ECOLOGY AND ABUNDANCE OF HYMENOXYS TEXANA (ASTERACEAE) JASON R. SINGHURST Wildlife Diversity Program Texas Parks & Wildlife Department 4200 Smith School Road Austin, Texas 78744 [email protected] NANCY SHACKELFORD School of Environmental Studies University of Victoria P.O. Box 1700 STN CSC Victoria, B.C. V8W 2Y2, CANADA WESLEY NEWMAN Katy Prairie Conservancy Field Office 31950 Hebert Road Waller, Texas 77484 JEFFREY N. MINK Department of Biology McLennan Community College 1400 College Drive Waco, Texas 76708 WALTER C. HOLMES Department of Biology Baylor University Waco, Texas 76798-7388 ABSTRACT During 2009-2010, the ecology of Hymenoxys texana , Texas Prairie Dawn, was studied at three prairie sites in Harris County, Texas to determine population variations across various saline prairie microhabitats and whether a 1989 recovery plan proposed for the species has been succeeding. Associated plant species, plant community structure and abundance comparisons between years for H. texana were also assessed. Densities of H. texana varied between zero and 429 individual plants/m 2, with an average of approximately 83 plants/m 2. No overall significant differences in plot densities between sites were found, although a significant increase in H. texana occurred across sites from 2009 to 2010. Notable plant associations included Thurovia triflora, Chloris texensis, Valerianella florifera, Willkommia texana var. texana, Atriplex texana along with 12 other regionally restricted species for a total of 378 associated taxa. -

Recovery and the Endangered Species Act by Mark Klym
NatureEye on FALL 2013 A publication of the Wildlife Division — Getting Texans Involved Contentious Coalitions for a Conservation Conundrum An Endangered Bat with a By Donnie Frels Tequila Connection ome things just go together – biscuits and gravy, hot coffee on a cold morning, camp fires Page 3 S and old friends. It seems easy and comfortable to relate these associations, almost natural. Other things just don’t mix – Aggies and Longhorns, ex-wives and girlfriends, Seinfeld and Newman. The Endangered Species Act Helps Restore the Endangered Most folks would put hunters and birders in the latter category although I might disagree. Having Kemp’s Ridley Sea Turtle experienced both activities and associated with each group, I often hear of hunters enjoying the Page 5 art of bird watching, albeit most are novices, while sitting in a deer blind enjoying the antics of a cautious roadrunner or curious green jay. However, in my unscientific survey, it seems fewer birders T&E Fishes of Texas participate in hunting or appreciate the positive ecological impacts regulated hunting activities may Page 7 produce. In one particular instance, hunters will have a key role in the recovery efforts of an endan- gered Texas songbird. Texas Prairie Dawn Conservation Success When the Endangered Species Act (ESA) of 1973 was initially passed by the 93rd Congress and in Houstlandia signed by President Richard Nixon, it was met with some skepticism by suspicious landowners leery Page 8 of federal government intrusion in matters involving private property rights. Perhaps nowhere in Texas was this more apparent than the biologically diverse and ecologically sensitive Edwards From Fatmuckets Plateau ecoregion in Central Texas. -

Threatened and Endangered Species Habitat Assessment
CESI Project Number 36-17 April 2018 Threatened and Endangered Species Habitat Assessment Project 28-F Prepared for: Sander Engineering Corporation & North Harris County Regional Water Authority Threatened and Endangered Species Habitat Assessment Project 28-F CESI Project Number 36-17 April 2018 Prepared For Sander Engineering Corporation 2901 Wilcrest, Suite 500 Houston, Texas 77042 Project Manager Claire Garvin Report Author Ethan Pauling, Amanda Sankey Data Collection Ally Altemose, PWS, Ethan Pauling, Trevor Pattillo, Amanda Sankey Maps and Figures Trevor Pattillo, Greg Sevcik Ground Photographs Ally Altemose, PWS, Ethan Pauling, Amanda Sankey Report Reviewers Claire Garvin, Leslie Hollaway Prepared By 402 Teetshorn Street Houston, Texas 77009 713.868.1043 www.CrouchEnvironmental.com [email protected] About The Cover: A Common Green Darner (Anax junius) rests on a branch in Harris County, Texas. Contents Appendices ............................................................................................ ii Acronyms ............................................................................................. iii Executive Summary ................................................................................. 1 Introduction ........................................................................................... 2 Methodology .......................................................................................... 3 Federally Listed Species Review ..........................................................................