2019 Environmental Epidemiology of Autism Research Network (EEARN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proceedings of the 20Th International Seminar of the ISME Commission on Music in Special Education, Music Therapy, and Music Medicine
Proceedings of the 20th International Seminar of the ISME Commission on Music in Special Education, Music Therapy, and Music Medicine Faculdade de Artes do Paraná – FAP CuritiBa, Brazil 17-18 July 2014 Editor Melita Belgrave ©International Society for Music Education 2014 www.isme.org All abstracts presented at the 2014 ISME World Conference in Porto Alegre, Brazil, were peer refereed before inclusion in the Conference program. In addition, completed papers were fully (blind) refereed by a panel of international authorities before inclusion in the Seminar Proceedings. Editorial Board Melita Belgrave, Editor Jessie Chen Helen Farrell Markku Kaikkonen Bo Nilsson Lyn Schraer-Joiner National Library of Australia Cataloguing-in-Publication Author: ISME Commission on Music in Special Education, Music Therapy, and Music Medicine International Seminar (20th: 2014: Curitba, Brazil) Title: Proceedings of the 20th International Seminar of the Commission on Music in Special Education, Music Therapy, and Music Medicine, Curitiba, Brazil [electronic resource] / ISBN: 978-0-9942055-3-7 (ebook) Notes: Includes bibliographical references. Subjects: Music--Congresses. Music in education--Congresses. ISME Commission on Music in Special Education, Music Therapy, and Music Medicine Dewey Number: 780.7 ii The Conference Organizing Committee and ISME are grateful to the following people who provided expert, independent advice and who acted as referees for selecting papers and workshops for presentation at the 2014 ISME World Conference: Commissioners 2012-2014 -

Psychopathology, Families, and Culture: Autism
Psychopathology, Families, and Culture: Autism a, b c Raphael Bernier, PhD *, Alice Mao, MD , Jennifer Yen, MD KEYWORDS Autism spectrum disorders Culture Families Developmental disabilities BACKGROUND AND OVERVIEW Autism spectrum disorders (ASDs) are now considered to be the most common of the developmental disorders. However, the effect of cultural influences on the diagnosis and treatment of ASDs has received limited attention. The lengthy diagnostic processes, complicated treatment planning, and associated medical symptoms pose challenges to clinicians in considering the cultural influences on the disorder. Furthermore, cultural factors affecting diagnostic processes, adaptation of the family to having a child with autism, and treatment differences may have received less atten- tion because neurodevelopmental changes seem to be much more significant in contributing to the abnormal social interaction, behaviors, and communication prob- lems. Although symptoms of biologic disorders may be similar across cultures, symptom description, interpretation, and acceptance can vary tremendously. Despite the limited research data available at present, evidence suggests that culture does play a role. This article reviews the available literature on cultural differences in diagnosis, acceptance, and treatment of ASD. It is important to focus on both macrolevel cultural factors—factors at the dominant culture level that affect the people in that society and microlevel factors—factors at the family level that affect response to diagnosis or treatment choice. Both can play a role in the course and outcome of an individual with ASD. Macrolevel factors, such as the availability of services, societal acceptance of the disorder, and existence of national- and/or state-funded treatment options, The authors have nothing to disclose. -

Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children
ORIGINAL CONTRIBUTION Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children ˚ ´ Pal Suren, MD, MPH Importance Prenatal folic acid supplements reduce the risk of neural tube defects Christine Roth, MSc in children, but it has not been determined whether they protect against other neu- Michaeline Bresnahan, PhD rodevelopmental disorders. Margaretha Haugen, PhD Objective To examine the association between maternal use of prenatal folic acid supplements and subsequent risk of autism spectrum disorders (ASDs) (autistic disor- Mady Hornig, MD der, Asperger syndrome, pervasive developmental disorder–not otherwise specified Deborah Hirtz, MD [PDD-NOS]) in children. Kari Kveim Lie, MD Design, Setting, and Patients The study sample of 85 176 children was derived from the population-based, prospective Norwegian Mother and Child Cohort Study W. Ian Lipkin, MD (MoBa). The children were born in 2002-2008; by the end of follow-up on March 31, Per Magnus, MD, PhD 2012, the age range was 3.3 through 10.2 years (mean, 6.4 years). The exposure of Ted Reichborn-Kjennerud, MD, PhD primary interest was use of folic acid from 4 weeks before to 8 weeks after the start of pregnancy, defined as the first day of the last menstrual period before conception. Synnve Schjølberg, MSc Relative risks of ASDs were estimated by odds ratios (ORs) with 95% CIs in a logistic George Davey Smith, MD, DSc regression analysis. Analyses were adjusted for maternal education level, year of birth, and parity. Anne-Siri Øyen, PhD Main Outcome Measure Specialist-confirmed diagnosis of ASDs. Ezra Susser, MD, DrPH Results At the end of follow-up, 270 children in the study sample had been diag- Camilla Stoltenberg, MD, PhD nosed with ASDs: 114 with autistic disorder, 56 with Asperger syndrome, and 100 with PDD-NOS. -

Lower Autism Risk with Folic Acid Supplements in Pregnancy 12 February 2013
Lower autism risk with folic acid supplements in pregnancy 12 February 2013 Women who took folic acid supplements in early of folic acid supplements in the mother during pregnancy almost halved the risk of having a child pregnancy and a reduced risk of childhood autism. with autism. Beginning to take folic acid supplements later in pregnancy did not reduce the "The study does not prove that folic acid risk. This is shown in new findings from the ABC supplements can prevent childhood autism. Study and Norwegian Mother and Child Cohort However, the findings are so apparent that they Study published in the Journal of The American constitute a good argument to further examine Medical Association (JAMA). possible causal mechanisms. It should also be ascertained whether folic acid is associated with a Women who took folic acid supplements from four reduced risk of other brain disorders in children," weeks before conception to eight weeks into says Surén. pregnancy had a 40 per cent lower risk of giving birth to children with childhood autism (classic Emphasises the importance of folic acid autism). Use of folic acid supplements midway supplements through pregnancy (week 22) had no effect. The results support the Norwegian Directorate of The findings only apply to a lower risk of childhood Health's recommendations for folic acid autism, the most severe form of autism. The supplements during pregnancy and emphasise the results show no reduction in the risk of atypical or importance of starting early—preferably before unspecific autism. The study also investigated the conception. prevalence of Asperger syndrome, but the number of examined children was too low to give a reliable Method result. -

Environmental and Genetic Factors in Autism Spectrum Disorders: Special Emphasis on Data from Arabian Studies
International Journal of Environmental Research and Public Health Review Environmental and Genetic Factors in Autism Spectrum Disorders: Special Emphasis on Data from Arabian Studies Noor B. Almandil 1,† , Deem N. Alkuroud 2,†, Sayed AbdulAzeez 2, Abdulla AlSulaiman 3, Abdelhamid Elaissari 4 and J. Francis Borgio 2,* 1 Department of Clinical Pharmacy Research, Institute for Research and Medical Consultation (IRMC), Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia; [email protected] 2 Department of Genetic Research, Institute for Research and Medical Consultation (IRMC), Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia; [email protected] (D.N.A.); [email protected] (S.A.) 3 Department of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia; [email protected] or [email protected] 4 Univ Lyon, University Claude Bernard Lyon-1, CNRS, LAGEP-UMR 5007, F-69622 Lyon, France; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +966-13-333-0864 † These authors contributed equally to this work. Received: 26 January 2019; Accepted: 19 February 2019; Published: 23 February 2019 Abstract: One of the most common neurodevelopmental disorders worldwide is autism spectrum disorder (ASD), which is characterized by language delay, impaired communication interactions, and repetitive patterns of behavior caused by environmental and genetic factors. This review aims to provide a comprehensive survey of recently published literature on ASD and especially novel insights into excitatory synaptic transmission. Even though numerous genes have been discovered that play roles in ASD, a good understanding of the pathophysiologic process of ASD is still lacking. -

Epidemiology Report of Autism in California
Report to the Legislature on the Principal Findings from The Epidemiology of Autism in California: A Comprehensive Pilot Study IN CALIFORNIA AUTISM M.I.N.D. Institute University of California, Davis October 17, 2002 THE EPIDEMIOLOGY OF UC Davis Project Staff: ○○○○○○○○○○○○○○○○○○○○○○○○○○○○○ Robert S. Byrd, M.D., M.P.H. Principal Investigator Allyson C. Sage, R.N., M.P.H. Project Manager Janet Keyzer, R.N.C., M.P.A. Lead Chart Abstractor Rachel Shefelbine Research Assistant/ADI-R Administrator Kasie Gee, M.P.H. Research Assistant Kristen Enders Administrative Assistant Jonathan Neufeld, Ph.D. Clinical Psychologist Nhue Do Post Graduate Researcher/ADI-R Administrator Kelly Heung, M.S. Post Graduate Researcher/ADI-R Administrator IN CALIFORNIA Tiffany Hughes Post Graduate Researcher Ruth Baron, R.N. Chart Abstractor Andrea Moore, M.S.W. Chart Abstractor Bonnie Walbridge, R.N., M.P.H. Chart Abstractor/ADI-R Administrator Steven Samuels, Ph.D. Statistical Consultant Daniel Tancredi, M.S. Statistical Consultant Jingsong He, M.S. Statistician Emma Calvert Student Assistant Diana Chavez Student Assistant Anna Elsdon Student Assistant Lolly Lee Student Assistant Denise Wong Student Assistant UCLA Site Project Staff: ○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○ AUTISM Marian Sigman, Ph.D. Co-Investigator Michael Bono, Ph.D. Co-Investigator Caitlin Beck Project Manager/ADI-R Administrator Sara Clavell Research Assistant/ADI-R Administrator Elizabeth Lizaola Research Assistant/ADI-R Administrator Vera Meyerkova Research Assistant/ADI-R Administrator THE EPIDEMIOLOGY -

Global Data on Autism Spectrum Disorders Prevalence: a Review of Facts, Fallacies and Limitations
Universal Journal of Clinical Medicine 5(2): 14-23, 2017 http://www.hrpub.org DOI: 10.13189/ujcm.2017.050202 Global Data on Autism Spectrum Disorders Prevalence: A Review of Facts, Fallacies and Limitations Onaolapo A Y1, Onaolapo O J2,* 1Behavioural Neuroscience/Neurobiology Unit, Department of Anatomy, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria 2Behavioural Neuroscience/Neuropharmacology Unit, Department of Pharmacology, Ladoke Akintola University of Technology, Osogbo, Osun State, Nigeria Copyright©2017 by authors, all rights reserved. Authors agree that this article remains permanently open access under the terms of the Creative Commons Attribution License 4.0 International License Abstract Autism spectrum disorders (ASDs) are a in the early to mid-1960s [2, 3], while the first range of neurodevelopmental disorders whose aetiologies hospital-based survey in sub-Saharan Africa was are largely unknown. In the past few decades, studies have conducted in five countries in the late 1970s [4]. Autism demonstrated that ASDs occur globally, and that the surveys have since become global, with different countries numbers of recorded cases are rising; however, conducting surveys to determine the prevalence and risk determining the true prevalence figures is still a major factors in their communities [1, 5], and thereby propose challenge, especially in developing nations. Also, subtle strategies to improve identification, diagnosis, differences in cultural norms might impede timely/accurate management as well as promote policy reforms that bring diagnosis and categorisation of patients. In this review, we awareness to the plight of the autistic child. examine the globally-available data on the prevalence of The term ‘Autism’ derives from the Greek word "autos", ASD and discuss some of the challenges of data acquisition, meaning "self”; and it was in the year 1911 that Eugen with reference to how these may impact the reliability of Bleuler first used the word autism in describing a symptom figures obtained. -

The Diagnosis of Autism: from Kanner to DSM‑III to DSM‑5 and Beyond
Journal of Autism and Developmental Disorders https://doi.org/10.1007/s10803-021-04904-1 S:I AUTISM IN REVIEW: 1980-2020: 40 YEARS AFTER DSM-III The Diagnosis of Autism: From Kanner to DSM‑III to DSM‑5 and Beyond Nicole E. Rosen1 · Catherine Lord1 · Fred R. Volkmar2,3 Accepted: 27 January 2021 © The Author(s) 2021 Abstract In this paper we review the impact of DSM-III and its successors on the feld of autism—both in terms of clinical work and research. We summarize the events leading up to the inclusion of autism as a “new” ofcial diagnostic category in DSM-III, the subsequent revisions of the DSM, and the impact of the ofcial recognition of autism on research. We discuss the uses of categorical vs. dimensional approaches and the continuing tensions around broad vs. narrow views of autism. We also note some areas of current controversy and directions for the future. Keywords Autism · History · Dimensional · Categorical · DSM It has now been nearly 80 years since Leo Kanner’s (1943) frst described by Kanner has changed across the past few classic description of infantile autism. Ofcial recognition of decades. When we refer to the concept in general, we will this condition took almost 40 years; several lines of evidence use the term autism, and when we refer to particular, ear- became available in the 1970s that demonstrated the valid- lier diagnostic constructs, we will use more specifc terms ity of the diagnostic concept, clarifed early misperceptions like autism spectrum disorder, infantile autism, and autistic about autism, and illustrated the need for clearer approaches disorder. -

Environmental Health Issue
FIFTH EDITION 2006, Volume 45 R5 Environmental Health and Autism In thIs Issue: Time To GeT a Grip By martha r. Herbert, m.D., ph.D. Beyond Behavior—Biomedical Diagnoses in Autism spectrum Disorders By Margaret L. Bauman, M.D. transforming the Public Debate on neurotoxicants By Elise Miller, M.Ed. ADVERTISEMENT ADVERTISEMENT Autism does not have to be a life sentence You’re not about to give up on your child. Neither Are We. Since , the Autism Treatment Center of America™ has provided innovative training programs for parents and professionals caringifor children challenged by Autism Spectrum Disorders and related developmental difficulties. • Practical Tools • Powerful Results • Limitless Hope c Help your child improve in all areas of over p learning, development, communication and hoto skill acquisition. : © W I Join us for our internationally-acclaimed ll T ERR Son-Rise Program® Start-Up, a y comprehensive weeklong training program for parents and professionals. We don’t put limits on the possibilities for your child. Free 25-Minute Initial Call 877-766-7473 We’ll give you the keys to unlock their world. HOME OF THE SON-RISE PROGRAM® SINCE 1983 South Undermountain Road Sheffield, MA - USA Telephone: -- • E-mail: [email protected] www.autismtreatment.com Copyright © 2006 by The Option Institute & Fellowship. All rights reserved. 02.06-6 CONTENTS December 2006 page 18 SpOTlIGHT Time to Get A Grip By marTHa r. HerBerT, m.D., pH.D. Does an environmental role in autism make sense? How do we decide? And if environment is involved in autism, what do we do about it? These are challenging questions. -

Sensitivity and Specificity of Early Screening for Autism
BJPsych Open (2019) 5, e41, 1–8. doi: 10.1192/bjo.2019.34 Sensitivity and specificity of early screening for autism Pål Surén, Alexandra Saasen-Havdahl, Michaeline Bresnahan, Deborah Hirtz, Mady Hornig, Catherine Lord, Ted Reichborn-Kjennerud, Synnve Schjølberg, Anne-Siri Øyen, Per Magnus, Ezra Susser, W. Ian Lipkin and Camilla Stoltenberg Background (95% CI 58–79) for ASD without phrase speech and 34% (95% CI – Early identification and diagnosis is beneficial for children with 29 40) for ASD with phrase speech. Specificity was then reduced – autism spectrum disorder (ASD). Universal early screening is to 89% (95% CI 89 90). recommended by many experts, but disputed because evidence is limited, and sensitivity and specificity in general populations Conclusions are largely unknown. Early ASD screening with a parent checklist had low sensitivity. It identified mainly individuals with ASD with significant develop- Aims mental delay and captured very few children with ASD with To estimate the sensitivity and specificity of early population- cognitive skills in the normal range. Increasing sensitivity was not based screening for ASDs. possible without severely compromising specificity. Method Declaration of interest The study was based on the Norwegian Mother and Child Cohort C.L. receives royalty for the Social Communication Study. The 36-month cohort questionnaire included the Social Questionnaire, which she has co-authored. Communication Questionnaire (SCQ), a 40-item screening instrument for ASD. Keywords Results Autistic spectrum disorders; developmental disorders; A total of 58 520 mothers (58%) responded to the questionnaire. epidemiology. By the end of follow-up on 31 December 2015, 385 (0.7%) indi- viduals with ASD had been identified among the responders’ Copyright and usage children. -

Prenatal Exposure to Antipsychotic Medication Does Not Increase Odds of Autism, ADHD
Spectrum | Autism Research News https://www.spectrumnews.org NEWS Prenatal exposure to antipsychotic medication does not increase odds of autism, ADHD BY PETER HESS 20 AUGUST 2021 Listen to this story: Children born to mothers who take antipsychotic medications during pregnancy do not have elevated odds of autism or attention deficit hyperactivity disorder (ADHD), nor are they more likely to be born preterm or underweight, according to a study released this past Monday in JAMA Internal Medicine. Some women with schizophrenia, Tourette syndrome or bipolar disorder take antipsychotic drugs, such as aripiprazole, haloperidol or risperidone. Clinicians have long debated whether women should discontinue these medications during pregnancy out of concern for the drugs’ effects on the developing fetus. But children born to mothers who take antipsychotics during pregnancy and to those who do not take them have similar outcomes, the new work shows. “Our findings do not support a recommendation for women to discontinue their regular antipsychotic treatment during pregnancy,” says senior investigator Kenneth Man, research fellow at the University College London School of Pharmacy in the United Kingdom. Prescribing antipsychotics during pregnancy can help prevent potentially dangerous psychotic episodes and ensure that an expectant mother can take care of herself, says Mady Hornig, associate professor of epidemiology at Columbia University, who was not involved in the study. “We certainly don’t want to be cavalier about the use of any medication during pregnancy, but one 1 / 2 Spectrum | Autism Research News https://www.spectrumnews.org also wants to balance out the implications of not treating.” Any apparent effects of antipsychotics on a developing fetus are likely due to the condition being treated, rather than the treatment, the study shows. -
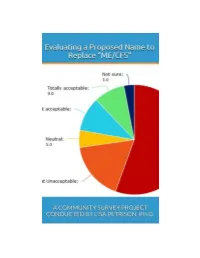
Read the Full Survey Report
Evaluating a Proposed Name to Replace ‘ME/CFS’: A Community Survey Project Conducted by Lisa Petrison, Ph.D. Published by Paradigm Change (March 2015) Copyright 2015 www.paradigmchange.me 2 Table of Contents Executive Summary ...................................................................................................................................... 5 Part 1 – Results Overview .......................................................................................................................... 13 Part 2 - Implications ................................................................................................................................... 29 Part 3 – Survey Questions .......................................................................................................................... 49 Part 4 – Main Survey Results ..................................................................................................................... 66 Part 5 – ME vs. Non-ME Results ............................................................................................................... 110 Part 6 – US ME vs. Non-ME Results ......................................................................................................... 127 Part 7 – Related Illnesses Results ............................................................................................................ 161 Part 8 – US vs. Non-US Results ................................................................................................................. 181