Natural Hybridization and Genetic and Morphological Variation Between Two Epiphytic Bromeliads
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollinators Drive Floral Evolution in an Atlantic Forest Genus Beatriz Neves1,2, Igor M
Copyedited by: AS AoB PLANTS 2020, Vol. 12, No. 5 doi:10.1093/aobpla/plaa046 Advance Access Publication August 22, 2020 Studies STUDIES Pollinators drive floral evolution in an Atlantic Forest genus Beatriz Neves1,2, Igor M. Kessous1, Ricardo L. Moura1, Dayvid R. Couto1, Camila M. Zanella3, Alexandre Antonelli2,4,5, Christine D. Bacon2,5, Fabiano Salgueiro6 and Andrea F. Costa7*, 1Universidade Federal do Rio de Janeiro, Museu Nacional, Programa de Pós Graduação em Ciências Biológicas (Botânica), Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil, 2Gothenburg Global Biodiversity Centre, Carl Skottsbergs Gata 22B, SE 41319 Gothenburg, Sweden, 3National Institute of Agricultural Botany, Huntingdon Road, Cambridge CB30LE, UK, 4Royal Botanic Gardens, Kew, Richmond TW9 3AE, Surrey, UK, 5Department of Biological and Environmental Sciences, University of Gothenburg, Carl Skottsbergs Gata 22B, SE 41319 Gothenburg, Sweden, 6Departamento de Botânica, Universidade Federal do Estado do Rio de Janeiro, Av. Pasteur, 458, 22290-240 Rio de Janeiro, RJ, Brazil, 7Departamento de Botânica, Universidade Federal do Rio de Janeiro, Museu Nacional, Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil *Corresponding author’s e-mail address: [email protected] Associate Editor: Karina Boege Abstract Pollinators are important drivers of angiosperm diversification at both micro- and macroevolutionary scales. Both hummingbirds and bats pollinate the species-rich and morphologically diverse genus Vriesea across its distribution in the Brazilian Atlantic Forest. Here, we (i) determine if floral traits predict functional groups of pollinators as documented, confirming the pollination syndromes in Vriesea and (ii) test if genetic structure in Vriesea is driven by geography (latitudinal and altitudinal heterogeneity) or ecology (pollination syndromes). -

Network Scan Data
Selbyana 15: 132-149 CHECKLIST OF VENEZUELAN BROMELIACEAE WITH NOTES ON SPECIES DISTRIBUTION BY STATE AND LEVELS OF ENDEMISM BRUCE K. HOLST Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, USA ABSTRACf. A checklist of the 24 genera and 364 native species ofBromeliaceae known from Venezuela is presented, including their occurrence by state and indications of which are endemic to the country. A comparison of the number of genera and species known from Mesoamerica (southern Mexico to Panama), Colombia, Venezuela, the Guianas (Guyana, Suriname, French Guiana), Ecuador, and Peru is presented, as well as a summary of the number of species and endemic species in each Venezuelan state. RESUMEN. Se presenta un listado de los 24 generos y 364 especies nativas de Bromeliaceae que se conocen de Venezuela, junto con sus distribuciones por estado y una indicaci6n cuales son endemicas a Venezuela. Se presenta tambien una comparaci6n del numero de los generos y especies de Mesoamerica (sur de Mexico a Panama), Colombia, Venezuela, las Guayanas (Guyana, Suriname, Guyana Francesa), Ecuador, y Peru, y un resumen del numero de especies y numero de especies endemicas de cada estado de Venezuela. INTRODUCTION Bromeliaceae (Smith 1971), and Revision of the Guayana Highland Bromeliaceae (Smith 1986). The checklist ofVenezuelan Bromeliaceae pre Several additional country records were reported sented below (Appendix 1) adds three genera in works by Smith and Read (1982), Luther (Brewcaria, Neoregelia, and Steyerbromelia) and (1984), Morillo (1986), and Oliva-Esteva and 71 species to the totals for the country since the Steyermark (1987). Author abbreviations used last summary of Venezuelan bromeliads in the in the checklist follow Brummit and Powell Flora de Venezuela series which contained 293 (1992). -

Ana Maria Benko-Iseppon, Marccus Alves & Rafael Louzada
Rodriguésia 66(2): A1-A66. 2015 http://rodriguesia.jbrj.gov.br DOI: 10.1590/2175-7860201566228 An overview and abstracts of the First World Congress on Bromeliaceae Evolution Ana Maria Benko-Iseppon, Marccus Alves & Rafael Louzada Abstracts of the Conferences, Symposia, Oral Presentations and Poster Presentations performed during the 1st World Congress on Bromeliaceae Evolution, March 2015 (Brazil): Reactive oxygen species and antioxidant enzyme activities in leaves of Guzmania monostachia plants under water deficit Abreu, Maria Elizabeth1; Carvalho, Victória2 & Mercier, Helenice1 CAM plants have the capacity to deal with highly changing environments due to the flexibility of reversible morphological and physiological adaptations to multiple stresses. However, little is known about the signalling pathway of ROS in plants with CAM metabolism, other than the knowledge that ROS production is limited in CAM plants. In the present study, we assessed the effects of drought stress on reactive oxygen species and antioxidant enzyme activities in leaf portions of Guzmania monostachia. The exposure of G. monostachia plants to 10 days of water deficit led to a decrease in the leaf relative water content (RWC) from 75% to 50% in all leaf portions (apical, middle and basal); hence, it was concluded that plants subjected to drought produced higher levels of reactive oxygen species (ROS) when compared with control plants. Significant variations to the formation of ROS were also identified in all leaf portions during the diurnal cycle. After ten days of CAM induction, H2O2 concentration increased significantly in contrast to control plants during the day-night cycle. In addition, the activity of antioxidant enzymes in processes related to the elimination of ROS was also evaluated. -

The Effects of Human Disturbance on Vascular Epiphyte in the Brazilian Atlantic Forest
The effects of human disturbance on vascular epiphyte in the Brazilian Atlantic Forest Edicson Parra-Sanchez Imperial College London Department of Life Science Silwood Park Campus Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) April 2018 1 “What we have to practice today is solidarity, one must not approach people to say Here we come to give the charity of our presence, to teach our science, to show you your mistakes, your ignorance, your lack of basics. We go with investigative zeal, and humble spirit, to learn in great source of wisdom which is the people.” Che Guevara 2 Declaration The data collection and work presented in this thesis is all my own. Dr Cristina Banks-Leite did provide guidance throughout my thesis. Chapters are organized as papers, and I used “we” to recognize the role of my supervisor Dr Cristina. Data sources and software are referenced along the text. The support of different people is acknowledge as follows: Data collection was done with field assistants Jordy Jerez, Manon Czuckermand and Tiago Gloria. The study design was done by ECOFOR (Biodiversity and Ecosystem Functioning in Degraded and Recovering Amazonian and Atlantic Forests). Taxa identification was done with the help of MSc Gabriel Marcusso (Piperaceae), Dr Carlos Nunes and Eric Hagsater (Orchidaceae), Ms Suzana Martins and Marcio Leofegario (Bromeliaceae), Dr Alain Chautems (Gesneriaceae), and Thomas Croat (Ferns). In chapter 2, advice on the model fitting and interpretation was provided by Dr Jack Hatfield. In chapter 3, advice on the interpretation of the n-dimension analysis was provided by Dr Thomas Guillerme (as author of the “dispRity” R package). -
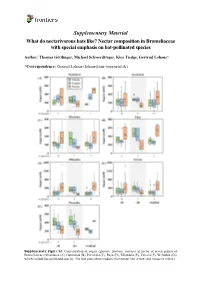
Supplementary Material What Do Nectarivorous Bats Like? Nectar Composition in Bromeliaceae with Special Emphasis on Bat-Pollinated Species
Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S1: Concentration of sugars (glucose, fructose, sucrose) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)) which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S2: Concentration of amino acids (ala, arg, asn, asp, gaba, gln, glu, gly, his, iso, leu, lys, met, phe, pro, ser, thr, trp, tyr, val) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S3: Cation concentrations (Ca2+, K+, Na+, Mg2+) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). -

Natural Hybridization and Genetic and Morphological Variation Between Two Epiphytic Bromeliads
Research Article Natural hybridization and genetic and morphological variation between two epiphytic bromeliads Jordana Neri1, Tânia Wendt2 and Clarisse Palma-Silva3* 1Programa de Pós Graduação em Botânica, Departamento de Botânica, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil 2Departamento de Botânica, Instituto de Biologia, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, RJ, Brazil 3Programa de Pós Graduação em Ecologia, Departamento de Ecologia – Universidade Estadual Paulista Julio Mesquita Filho, Avenida 24A 1515, 13506-900 Rio Claro, SP, Brazil Received: 23 March 2017 Editorial decision: 14 October 2017 Accepted: 22 November 2017 Published: 27 November 2017 Associate Editor: Adrian C. Brennan Citation: Neri J, Wendt T, Palma-Silva C. 2018. Natural hybridization and genetic and morphological variation between two epiphytic bromeliads. AoB PLANTS 10: plx061; doi: 10.1093/aobpla/plx061 Abstract. Reproductive isolation is of fundamental importance for maintaining species boundaries in sympatry. Here, we examine the genetic and morphological differences between two closely related bromeliad species: Vriesea simplex and Vriesea scalaris. Furthermore, we examined the occurrence of natural hybridization and discuss the action of reproductive isolation barriers. Nuclear genomic admixture suggests hybridization in sympatric popula- tions, although interspecific gene flow is low among species in all sympatric zones (Nem < 0.5). Thus, morphological and genetic divergence (10.99 %) between species can be maintained despite ongoing natural hybridization. Cross- evaluation of our genetic and morphological data suggests that species integrity is maintained by the simultaneous action of multiple barriers, such as divergent reproductive systems among species, differences in floral traits and low hybrid seed viability. -

Revista Mexicana De Biodiversidad
Revista Mexicana de Biodiversidad Revista Mexicana de Biodiversidad 91 (2020): e913090 Ecology Genetic diversity and population structure of the Chilean native, Tillandsia landbeckii (Bromeliaceae), from the Atacama Desert Diversidad genética y estructura poblacional de Tillandsia landbeckii (Bromeliaceae), nativa chilena del desierto de Atacama Elizabeth Bastías a, Edith Choque-Ayaviri b, Joel Flores c, Glenda Fuentes-Arce d, Patricio López-Sepúlveda d, Wilson Huanca-Mamani b, * a Laboratorio de Fisiología Vegetal, Departamento de Producción Agrícola, Facultad de Ciencias Agronómicas, Universidad de Tarapacá, Casilla 6-D, Arica, Chile b Laboratorio de Biología Molecular de Plantas, Departamento de Producción Agrícola, Facultad de Ciencias Agronómicas, Universidad de Tarapacá, Casilla 6-D, Arica, Chile c Laboratorio de Genómica y Bioinformática del Instituto de Biotecnología, Universidad Nacional Agraria La Molina, Av. La Molina s/n, Lima, Peru d Departamento de Botánica, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Casilla 160-C, Concepción, Chile *Corresponding author: [email protected] (W. Huanca-Mamani) Received: 3 June 2019; accepted: 17 February 2020 Abstract Tillandsia landbeckii Phil. is a typical plant of the Atacama Desert in the north of Chile. There is no genetic data available at the population level for this species, and this information is critical for developing and implementing effective conservation measures. In this study, we investigated for the first time the genetic diversity and population structure in 2 natural populations of T. landbeckii using AFLP markers. Seven primer combinations produced 405 bands and of them, 188 (46.42%) were polymorphic. The Pampa Dos Cruces population (Pp = 88.30%, He = 0.327, and I = 0.483) showed a higher genetic diversity level than Pampa Camarones population (Pp = 71.28%, He = 0.253, and I = 0.380). -

Vellozo) Lb Smith (Bromeliaceae
Pak. J. Bot ., 46(6): 2179-2187, 2014. DIRECT ORGANOGENESIS AND LEAF-ANATOMY MODIFICATIONS IN VITRO OF NEOREGELIA CONCENTRICA (VELLOZO) L.B. SMITH (BROMELIACEAE) JOÃO PAULO RODRIGUES MARTINS 1* , EDILSON ROMAIS SCHMILDT 2, RODRIGO SOBREIRA ALEXANDRE 2, EVARISTO MAURO DE CASTRO 1, THAÍS FURTADO NANI 1, MARINÊS FERREIRA PIRES 1AND MOACIR PASQUAL 1 1Federal University of Lavras, Biology Department, Lavras, MG, Brazil 2Federal University of Espírito Santo, Agricultural and Biological Sciences Department, São Mateus, ES, Brazil *Corresponding g author’s e-mail: [email protected]; + 55353829-1783 Abstract Tissue culture can contribute in the multiplication of several species with commercial interest, like the bromeliads. It was aimed to evaluate cytokinins and its concentrations in the multiplication and leaf structure of Neoregelia concentrica (Vellozo) L.B. Smith. Previously In vitro -established N. concentrica plants were inoculated in MS medium supplemented with 6-benzylaminopurine (BAP) or Kinetin (KIN) with concentrations 0.0, 5.0, 10.0 and 15.0 µM. For the anatomic analyses tree plants of each treatment were randomly sampled at 60-day growth. Significant differences were verified in the evaluated characteristics due to the treatments. The raise in cytokinin concentrations induced a higher percentage and average number of explants with shoots. BAP provided higher averages when compared to KIN. The cytokinin use modified the epidermal structure and induced a larger thickening of the water-storage and chlorophyll parenchymas. The use of 15.0 µM BAP was efficient in the In vitro multiplication and in the leaf tissue development of N. concentrica. Key words: Bromeliad, Cytokinin, In vitro multiplication, Tissue culture. -

Interação Entre Epífitas Vasculares E Forófitos: Estrutura E Padrões De Distribuição
INTERAÇÃO ENTRE EPÍFITAS VASCULARES E FORÓFITOS: ESTRUTURA E PADRÕES DE DISTRIBUIÇÃO TALITHA MAYUMI FRANCISCO UNIVERSIDADE ESTADUAL DO NORTE FLUMINENSE DARCY RIBEIRO – UENF CAMPOS DOS GOYTACAZES – RJ JUNHO DE 2017 INTERAÇÃO ENTRE EPÍFITAS VASCULARES E FORÓFITOS: ESTRUTURA E PADRÕES DE DISTRIBUIÇÃO TALITHA MAYUMI FRANCISCO Tese apresentada ao Centro de Biociências e Biotecnologia da Universidade Estadual do Norte Fluminense Darcy Ribeiro, como parte das exigências para obtenção do título de Doutor em Ecologia e Recursos Naturais. Orientador: PhD Carlos Rámon Ruiz-Miranda Universidade Estadual do Norte Fluminense Darcy Ribeiro, Centro de Biociências e Biotecnologia, Laboratório de Ciências Ambientais Coorientador: Dr. Mário Luís Garbin Universidade de Vila Velha, Laboratório de Ecologia Vegetal CAMPOS DOS GOYTACAZES – RJ JUNHO DE 2017 II III Dedico... A minha família, em especial a minha querida e amada mãe Edina e minha madrinha Célia (in memoriam) por serem os maiores e melhores exemplos de vida. E ao Dayvid, fonte inesgotável de amor. IV AGRADECIMENTOS Essa fase que aqui se encera foi um dos desafios mais imponente que a vida me proporcionou. Nessa fase, pude ultrapassar as barreiras da inércia, sair da “zona de conforto” e adentrar em um mundo tão vastos de informações novas. Não foi nada fácil, confesso! Mas, levo a certeza que somos capazes de vencer todos esses obstáculos que nos são apresentados para alcançarmos voos ainda mais altos. Por isso, gratidão pela oportunidade, vida! Agradeço, À Universidade Estadual do Norte Fluminense Darcy Ribeiro e ao Programa de Pós- Graduação em Ecologia e Recursos Naturais pela oportunidade de realização do curso de doutorado. À Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES pela concessão de bolsa de estudos durante o período de vigência do doutorado. -

Induction of in Vitro Shoots of Billbergia Euphemiae E. Morren (Bromeliaceae) from Leaf Explants
Acta Scientiarum http://www.uem.br/acta ISSN printed: 1679-9283 ISSN on-line: 1807-863X Doi: 10.4025/actascibiolsci.v38i2.31694 Induction of in vitro shoots of Billbergia euphemiae E. Morren (Bromeliaceae) from leaf explants Mariela Justiniano Simão1*, Daniele Vidal Faria2, Elias Terra Werner3, Taís Cristina Bastos Soares3 and Andreia Barcelos Passos Lima Gontijo4 1Núcleo de Biotecnologia Vegetal, Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier, 524 PHLC, 20550-013, Rio de Janeiro, Rio de Janeiro, Brazil. 2Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil. 3Universidade Federal do Espírito Santo, Centro de Ciências Agrárias, Alegre, Espírito Santo, Brazil. 4Universidade Federal do Espírito Santo, Centro Universitário Norte do Espírito Santo, Departamento de Ciências Agrárias e Biológicas, São Mateus, Espírito Santo, Brazil. *Author for correspondence. E-mail: [email protected] ABSTRACT. Bromeliads are an important group for the maintenance of the Atlantic Forest, with many threatened species due to exacerbated extraction and destruction of their natural habitats. Considering the need of developing protocols for the conservation of these species, the aim of this work was to evaluate the effect of different growth regulators in the in vitro induction of shoots of Billbergia euphemiae. Leaf explants were excised from seedlings derived from in vitro germination and grown on MS medium supplemented with NAA (0, 1 or 2 μM) and BA (0, 2, 4 or 6 μM) combinations. The evaluation of the number of shoots per explant, shoot length, number of leaves per shoot and longest leaf length average was carried out after 30 and 60 days of culture. -

Nuclear Genes, Matk and the Phylogeny of the Poales
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2018 Nuclear genes, matK and the phylogeny of the Poales Hochbach, Anne ; Linder, H Peter ; Röser, Martin Abstract: Phylogenetic relationships within the monocot order Poales have been well studied, but sev- eral unrelated questions remain. These include the relationships among the basal families in the order, family delimitations within the restiid clade, and the search for nuclear single-copy gene loci to test the relationships based on chloroplast loci. To this end two nuclear loci (PhyB, Topo6) were explored both at the ordinal level, and within the Bromeliaceae and the restiid clade. First, a plastid reference tree was inferred based on matK, using 140 taxa covering all APG IV families of Poales, and analyzed using parsimony, maximum likelihood and Bayesian methods. The trees inferred from matK closely approach the published phylogeny based on whole-plastome sequencing. Of the two nuclear loci, Topo6 supported a congruent, but much less resolved phylogeny. By contrast, PhyB indicated different phylo- genetic relationships, with, inter alia, Mayacaceae and Typhaceae sister to Poaceae, and Flagellariaceae in a basally branching position within the Poales. Within the restiid clade the differences between the three markers appear less serious. The Anarthria clade is first diverging in all analyses, followed by Restionoideae, Sporadanthoideae, Centrolepidoideae and Leptocarpoideae in the matK and Topo6 data, but in the PhyB data Centrolepidoideae diverges next, followed by a paraphyletic Restionoideae with a clade consisting of the monophyletic Sporadanthoideae and Leptocarpoideae nested within them. The Bromeliaceae phylogeny obtained from Topo6 is insufficiently sampled to make reliable statements, but indicates a good starting point for further investigations. -

Plant-Hummingbird Interactions: Natural History and Ecological Networks
Pietro Kiyoshi Maruyama Mendonça Plant-hummingbird interactions: natural history and ecological networks Interações entre plantas e beija-flores: história natural e redes ecológicas CAMPINAS 2015 Aos meus pais, Dario e Isumi e a Amandinha Agradecimentos Uma tese de doutorado é o resultado de um longo caminho, e inevitavelmente é preciso contar com a ajuda de muitas pessoas para ser finalizada. Tudo começou com os meus pais, Dario e Isumi, que resolveram "juntar as coisas" a quase trinta anos atrás. E a quase trinta anos eles têm feito de tudo para me dar todo o apoio possível. Agradeço muito a sorte de ter tido pais tão dedicados e amorosos. Obrigado! Essa tese não seria possível sem o apoio e orientação da Profa. Marlies. Agradeço o fato de ela ter me aberto as portas do seu laboratório e de certa maneira também da Unicamp. A professora é sempre um grande exemplo (que todos gostariam de ser) de como lidar com a vida acadêmica, para que esta continue divertida para você, mas também para os outros ao seu redor. Espero ter pego alguma coisa da sua sabedoria! Chegando aqui, ainda vejo que a minha formação se deve muito ao meu primeiro orientador, o Paulo. Em muitos aspectos parecido com a Profa. Marlies, mas em tantos outros bem diferente, é também um exemplo que tenho para mim. O que me deixa muito feliz é que as portas da sua sala continuam sempre abertas. Essa tese também me ofereceu a oportunidade de aprender com um co-orientador "gringo". Agradeço muito ao Bo, pelo crescimento profissional tão importante que me proporcionou.