Chapter 9 Chemical Bonding-II-Molecular Orbits And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

Transcription 12.02.15
Lecture 12A • 02/15/12 We’re going to continue our discussion of conjugation. If we go back and talk about electrons and how they act like waves, [there’s] something known as the particle in a box. You’ve got some kind of box where imagine that the walls are infinitely tall. It’s a one-dimensional system, where the electron, all it can do, is go left and right between the two walls of the box. The electron’s a wave; it can’t exist outside the box, so whatever function, whatever wave we use to describe the electron, has to have a value of zero at one end of the box and zero at the other end of the box. Graphically, what do the solutions look like? We have something like this, where we’re talking about energy potential; it’s infinite at either end of the box, and zero in between. We have an electron that’s bouncing around inside of it. An electron’s a wave, and there’s function that describes that wave. The only way it can fit in this box and physically make sense is if the wave starts and stops and the ends of the box. It turns out that – long, long, long story short – one of the solutions for the Schrödinger equation is just a sine function – actually, it’s part of an exponential version of a sine function. It’s something, at least, we can draw a pretty picture of. That’s why you’ll start with this problem in a discussion of quantum mechanics, because it is solvable, it is only on in dimension, and it’s more humanly possible to discuss. -

Aromaticity Sem- Ii
AROMATICITY SEM- II In 1931, German chemist and physicist Sir Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties .This is a very popular and useful rule to identify aromaticity in monocyclic conjugated compound. According to which a planar monocyclic conjugated system having ( 4n +2) delocalised (where, n = 0, 1, 2, .....) electrons are known as aromatic compound . For example: Benzene, Naphthalene, Furan, Pyrrole etc. Criteria for Aromaticity 1) The molecule is cyclic (a ring of atoms) 2) The molecule is planar (all atoms in the molecule lie in the same plane) 3) The molecule is fully conjugated (p orbitals at every atom in the ring) 4) The molecule has 4n+2 π electrons (n=0 or any positive integer Why 4n+2π Electrons? According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons. - This is true of aromatic compounds, meaning they are quite stable. - With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted by n), leaving all bonding orbitals filled and no anti-bonding orbitals occupied. This gives a total of 4n+2π electrons. - As for example: Benzene has 6π electrons. Its first 2π electrons fill the lowest energy orbital, and it has 4π electrons remaining. These 4 fill in the orbitals of the succeeding energy level. The criteria for Antiaromaticity are as follows: 1) The molecule must be cyclic and completely conjugated 2) The molecule must be planar. -

8.3 Bonding Theories >
8.3 Bonding Theories > Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Molecular Orbitals How are atomic and molecular orbitals related? 2 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • The model you have been using for covalent bonding assumes the orbitals are those of the individual atoms. • There is a quantum mechanical model of bonding, however, that describes the electrons in molecules using orbitals that exist only for groupings of atoms. 3 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • When two atoms combine, this model assumes that their atomic orbitals overlap to produce molecular orbitals, or orbitals that apply to the entire molecule. 4 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. • A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding orbital. 5 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Sigma Bonds When two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting two atomic nuclei, a sigma bond is formed. • Its symbol is the Greek letter sigma (σ). -

Molecular Orbital Theory
Molecular Orbital Theory The Lewis Structure approach provides an extremely simple method for determining the electronic structure of many molecules. It is a bit simplistic, however, and does have trouble predicting structures for a few molecules. Nevertheless, it gives a reasonable structure for many molecules and its simplicity to use makes it a very useful tool for chemists. A more general, but slightly more complicated approach is the Molecular Orbital Theory. This theory builds on the electron wave functions of Quantum Mechanics to describe chemical bonding. To understand MO Theory let's first review constructive and destructive interference of standing waves starting with the full constructive and destructive interference that occurs when standing waves overlap completely. When standing waves only partially overlap we get partial constructive and destructive interference. To see how we use these concepts in Molecular Orbital Theory, let's start with H2, the simplest of all molecules. The 1s orbitals of the H-atom are standing waves of the electron wavefunction. In Molecular Orbital Theory we view the bonding of the two H-atoms as partial constructive interference between standing wavefunctions of the 1s orbitals. The energy of the H2 molecule with the two electrons in the bonding orbital is lower by 435 kJ/mole than the combined energy of the two separate H-atoms. On the other hand, the energy of the H2 molecule with two electrons in the antibonding orbital is higher than two separate H-atoms. To show the relative energies we use diagrams like this: In the H2 molecule, the bonding and anti-bonding orbitals are called sigma orbitals (σ). -

Inorganic Chemistry/Chemical Bonding/MO Diagram 1
Inorganic Chemistry/Chemical Bonding/MO Diagram 1 Inorganic Chemistry/Chemical Bonding/MO Diagram A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular [1] [2] [3] . This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen and carbon monoxide but becomes more complex when discussing polynuclear molecules such as methane. It explains why some molecules exist and not others, how strong bonds are, and what electronic transitions take place. Dihydrogen MO diagram The smallest molecule, hydrogen gas exists as dihydrogen (H-H) with a single covalent bond between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital for its electron, the bond forms by overlap of these two atomic orbitals. In figure 1 the two atomic orbitals are depicted on the left and on the right. The vertical axis always represents the orbital energies. The atomic orbital energy correlates with electronegativity as a more electronegative atom holds an electron more tightly thus lowering its energy. MO treatment is only valid when the atomic orbitals have comparable energy; when they differ greatly the mode of bonding becomes ionic. Each orbital is singly occupied with the up and down arrows representing an electron. The two AO's can overlap in two ways depending on their phase relationship. The phase of an orbital is a direct consequence of the oscillating, wave-like properties of electrons. -

Structure of Benzene, Orbital Picture, Resonance in Benzene, Aromatic Characters, Huckel’S Rule B
Dr.Mrityunjay Banerjee, IPT, Salipur B PHARM 3rd SEMESTER BP301T PHARMACEUTICAL ORGANIC CHEMISTRY –II UNIT I UNIT I Benzene and its derivatives 10 Hours A. Analytical, synthetic and other evidences in the derivation of structure of benzene, Orbital picture, resonance in benzene, aromatic characters, Huckel’s rule B. Reactions of benzene - nitration, sulphonation, halogenationreactivity, Friedelcrafts alkylation- reactivity, limitations, Friedelcrafts acylation. C. Substituents, effect of substituents on reactivity and orientation of mono substituted benzene compounds towards electrophilic substitution reaction D. Structure and uses of DDT, Saccharin, BHC and Chloramine Benzene and its Derivatives Chemists have found it useful to divide all organic compounds into two broad classes: aliphatic compounds and aromatic compounds. The original meanings of the words "aliphatic" (fatty) and "aromatic" (fragrant/ pleasant smell). Aromatic compounds are benzene and compounds that resemble benzene in chemical behavior. Aromatic properties are those properties of benzene that distinguish it from aliphatic hydrocarbons. Benzene: A liquid that smells like gasoline Boils at 80°C & Freezes at 5.5°C It was formerly used to decaffeinate coffee and component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. A precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent in cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited. In 1970s it was associated with leukemia deaths. -

Alkenes and Alkynes
02/21/2019 CHAPTER FOUR Alkenes and Alkynes H N O I Cl C O C O Cl F3C C Cl C Cl Efavirenz Haloprogin (antiviral, AIDS therapeutic) (antifungal, antiseptic) Chapter 4 Table of Content * Unsaturated Hydrocarbons * Introduction and hybridization * Alkenes and Alkynes * Benzene and Phenyl groups * Structure of Alkenes, cis‐trans Isomerism * Nomenclature of Alkenes and Alkynes * Configuration cis/trans, and cis/trans Isomerism * Configuration E/Z * Physical Properties of Hydrocarbons * Acid‐Base Reactions of Hydrocarbons * pka and Hybridizations 1 02/21/2019 Unsaturated Hydrocarbons • Unsaturated Hydrocarbon: A hydrocarbon that contains one or more carbon‐carbon double or triple bonds or benzene‐like rings. – Alkene: contains a carbon‐carbon double bond and has the general formula CnH2n. – Alkyne: contains a carbon‐carbon triple bond and has the general formula CnH2n‐2. Introduction Alkenes ● Hydrocarbons containing C=C ● Old name: olefins • Steroids • Hormones • Biochemical regulators 2 02/21/2019 • Alkynes – Hydrocarbons containing C≡C – Common name: acetylenes Unsaturated Hydrocarbons • Arene: benzene and its derivatives (Ch 9) 3 02/21/2019 Benzene and Phenyl Groups • We do not study benzene and its derivatives until Chapter 9. – However, we show structural formulas of compounds containing a phenyl group before that time. – The phenyl group is not reactive under any of the conditions we describe in chapters 5‐8. Structure of Alkenes • The two carbon atoms of a double bond and the four atoms bonded to them lie in a plane, with bond angles of approximately 120°. 4 02/21/2019 Structure of Alkenes • Figure 4.1 According to the orbital overlap model, a double bond consists of one bond formed by overlap of sp2 hybrid orbitals and one bond formed by overlap of parallel 2p orbitals. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -
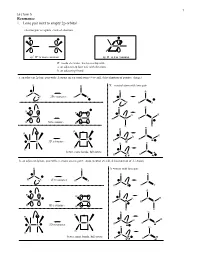
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚.