Perkinsus Marinus and P. Olseni/Atlanticus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Molecular Data and the Evolutionary History of Dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Un
Molecular data and the evolutionary history of dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Universitat Heidelberg, 1993 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES Department of Botany We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November 2003 © Juan Fernando Saldarriaga Echavarria, 2003 ABSTRACT New sequences of ribosomal and protein genes were combined with available morphological and paleontological data to produce a phylogenetic framework for dinoflagellates. The evolutionary history of some of the major morphological features of the group was then investigated in the light of that framework. Phylogenetic trees of dinoflagellates based on the small subunit ribosomal RNA gene (SSU) are generally poorly resolved but include many well- supported clades, and while combined analyses of SSU and LSU (large subunit ribosomal RNA) improve the support for several nodes, they are still generally unsatisfactory. Protein-gene based trees lack the degree of species representation necessary for meaningful in-group phylogenetic analyses, but do provide important insights to the phylogenetic position of dinoflagellates as a whole and on the identity of their close relatives. Molecular data agree with paleontology in suggesting an early evolutionary radiation of the group, but whereas paleontological data include only taxa with fossilizable cysts, the new data examined here establish that this radiation event included all dinokaryotic lineages, including athecate forms. Plastids were lost and replaced many times in dinoflagellates, a situation entirely unique for this group. Histones could well have been lost earlier in the lineage than previously assumed. -

Phylogenetic Relationships of the Genus Frenkelia
International Journal for Parasitology 29 (1999) 957±972 Phylogenetic relationships of the genus Frenkelia: a review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequencesp N.B. Mugridge a, D.A. Morrison a, A.M. Johnson a, K. Luton a, 1, J.P. Dubey b, J. Voty pka c, A.M. Tenter d, * aMolecular Parasitology Unit, University of Technology, Sydney NSW, Australia bUS Department of Agriculture, ARS, LPSI, PBEL, Beltsville MD, USA cDepartment of Parasitology, Charles University, Prague, Czech Republic dInstitut fuÈr Parasitologie, TieraÈrztliche Hochschule Hannover, BuÈnteweg 17, D-30559 Hannover, Germany Received 3 April 1999; accepted 3 May 1999 Abstract The dierent genera currently classi®ed into the family Sarcocystidae include parasites which are of signi®cant medical, veterinary and economic importance. The genus Sarcocystis is the largest within the family Sarcocystidae and consists of species which infect a broad range of animals including mammals, birds and reptiles. Frenkelia, another genus within this family, consists of parasites that use rodents as intermediate hosts and birds of prey as de®nitive hosts. Both genera follow an almost identical pattern of life cycle, and their life cycle stages are morphologically very similar. How- ever, the relationship between the two genera remains unresolved because previous analyses of phenotypic characters and of small subunit ribosomal ribonucleic acid gene sequences have questioned the validity of the genus Frenkelia or the monophyly of the genus Sarcocystis if Frenkelia was recognised as a valid genus. We therefore subjected the large subunit ribosomal ribonucleic acid gene sequences of representative taxa in these genera to phylogenetic analyses to ascertain a de®nitive relationship between the two genera. -

De Novo Transcriptome Assembly of Perkinsus Olseni Trophozoite Stimulated in Vitro with Manila Clam (Ruditapes Philippinarum) Plasma
Journal of Invertebrate Pathology 135 (2016) 22–33 Contents lists available at ScienceDirect Journal of Invertebrate Pathology journal homepage: www.elsevier.com/locate/jip De novo transcriptome assembly of Perkinsus olseni trophozoite stimulated in vitro with Manila clam (Ruditapes philippinarum) plasma Abul Farah Md. Hasanuzzaman a,b, Diego Robledo c, Antonio Gómez-Tato d, Jose A. Alvarez-Dios e, ⇑ Peter W. Harrison f, Asunción Cao g, Sergio Fernández-Boo g, Antonio Villalba g, Belén G. Pardo a, , Paulino Martínez a a Departamento de Xenética, Facultade de Veterinaria, Universidade de Santiago de Compostela, Lugo 27002, Spain b Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna 9208, Bangladesh c Departamento de Xenética, Facultade de Bioloxía, Universidade de Santiago de Compostela, Santiago de Compostela 15782, Spain d Departamento de Xeometría e Topoloxía, Facultade de Matemáticas, Universidade de Santiago de Compostela, Santiago de Compostela 15782, Spain e Departamento de Matemática Aplicada, Facultade de Matemáticas, Universidade de Santiago de Compostela, Santiago de Compostela 15782, Spain f Department of Genetics, Evolution and Environment, University College London, London WC1E 6BT, United Kingdom g Centro de Investigacións Mariñas (CIMA), Consellería do Medio Rural e do Mar, Xunta de Galicia, 36620 Vilanova de Arousa, Spain article info abstract Article history: The protistan parasite Perkinsus olseni is a deadly causative agent of perkinsosis, a molluscan disease Received 16 September 2015 affecting Manila clam (Ruditapes philippinarum), having a significant impact on world mollusc production. Revised 18 January 2016 Deciphering the underlying molecular mechanisms in R. philippinarum-P. olseni interaction is crucial for Accepted 24 January 2016 controlling this parasitosis. The present study investigated the transcriptional expression in the parasite Available online 25 January 2016 trophozoite using RNA-seq. -

Perkinsus Marinus Susceptibility and Defense-Related Activities in Eastern Oysters Crassostrea Virginica: Temperature Effects
DISEASES OF AQUATIC ORGANISMS Vol. 16: 223-234,1993 Published September 9 Dis. aquat. Org. Perkinsus marinus susceptibility and defense-related activities in eastern oysters Crassostrea virginica: temperature effects Fu-Lin E. Chu, Jerome F. La Peyre Virginia Institute of Marine Science, School of Marine Science, College of William and Mary, Gloucester Point, Virginia 23062, USA ABSTRACT. The relationship of potential defense-related cellular and humoral activities and the sus- ceptibility of eastern oysters Crassostrea virginica to the parasite Perkinsus marinus were examined at 10, 15, 20 and 25 "C. Oysters were acclimated at experimental temperatures for 20 d and then chal- lenged with R marinus. Total hemocyte counts (TC) and percentage of granulocytes (PG) 20 d after temperature acclimation were higher in oysters at high than at low acclimation temperature. Higher protein (P)and lysozyme (L) concentrations were found in oysters at 10 and 15 "C. No significant differ- ences in hemagglutination (H) titers due to temperature acclimation were observed. Infection preva- lence 46 d after challenge by R marinus was 100, 91, 46 and 23 % respectively, for oysters at 25, 20, 15 and 10 "C. Disease intensity increased with temperature. Oysters at higher temperatures had greater PG and TC and hemocyte phagocytic activity. No difference was found in TC and PG between control and challenged oysters within each temperature treatment. Bleeding may to some extent reduce TC and PG in oysters. P did not vary much among temperatures. No reduction of P in oysters was found due to P. marinuschallenge and infection. L tended to be higher in oysters at lower than at higher treat- ment temperatures. -

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -

University of Oklahoma
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By JOSHUA THOMAS COOPER Norman, Oklahoma 2017 MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION APPROVED FOR THE DEPARTMENT OF MICROBIOLOGY AND PLANT BIOLOGY BY ______________________________ Dr. Boris Wawrik, Chair ______________________________ Dr. J. Phil Gibson ______________________________ Dr. Anne K. Dunn ______________________________ Dr. John Paul Masly ______________________________ Dr. K. David Hambright ii © Copyright by JOSHUA THOMAS COOPER 2017 All Rights Reserved. iii Acknowledgments I would like to thank my two advisors Dr. Boris Wawrik and Dr. J. Phil Gibson for helping me become a better scientist and better educator. I would also like to thank my committee members Dr. Anne K. Dunn, Dr. K. David Hambright, and Dr. J.P. Masly for providing valuable inputs that lead me to carefully consider my research questions. I would also like to thank Dr. J.P. Masly for the opportunity to coauthor a book chapter on the speciation of diatoms. It is still such a privilege that you believed in me and my crazy diatom ideas to form a concise chapter in addition to learn your style of writing has been a benefit to my professional development. I’m also thankful for my first undergraduate research mentor, Dr. Miriam Steinitz-Kannan, now retired from Northern Kentucky University, who was the first to show the amazing wonders of pond scum. Who knew that studying diatoms and algae as an undergraduate would lead me all the way to a Ph.D. -

Symbiodinium Genomes Reveal Adaptive Evolution of Functions Related to Symbiosis
bioRxiv preprint doi: https://doi.org/10.1101/198762; this version posted October 5, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Article 2 Symbiodinium genomes reveal adaptive evolution of 3 functions related to symbiosis 4 Huanle Liu1, Timothy G. Stephens1, Raúl A. González-Pech1, Victor H. Beltran2, Bruno 5 Lapeyre3,4, Pim Bongaerts5, Ira Cooke3, David G. Bourne2,6, Sylvain Forêt7,*, David J. 6 Miller3, Madeleine J. H. van Oppen2,8, Christian R. Voolstra9, Mark A. Ragan1 and Cheong 7 Xin Chan1,10,† 8 1Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, 9 Australia 10 2Australian Institute of Marine Science, Townsville, QLD 4810, Australia 11 3ARC Centre of Excellence for Coral Reef Studies and Department of Molecular and Cell 12 Biology, James Cook University, Townsville, QLD 4811, Australia 13 4Laboratoire d’excellence CORAIL, Centre de Recherches Insulaires et Observatoire de 14 l’Environnement, Moorea 98729, French Polynesia 15 5Global Change Institute, The University of Queensland, Brisbane, QLD 4072, Australia 16 6College of Science and Engineering, James Cook University, Townsville, QLD 4811, 17 Australia 18 7Research School of Biology, Australian National University, Canberra, ACT 2601, Australia 19 8School of BioSciences, The University of Melbourne, VIC 3010, Australia 1 bioRxiv preprint doi: https://doi.org/10.1101/198762; this version posted October 5, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

A Parasite of Marine Rotifers: a New Lineage of Dinokaryotic Dinoflagellates (Dinophyceae)
Hindawi Publishing Corporation Journal of Marine Biology Volume 2015, Article ID 614609, 5 pages http://dx.doi.org/10.1155/2015/614609 Research Article A Parasite of Marine Rotifers: A New Lineage of Dinokaryotic Dinoflagellates (Dinophyceae) Fernando Gómez1 and Alf Skovgaard2 1 Laboratory of Plankton Systems, Oceanographic Institute, University of Sao˜ Paulo, Prac¸a do Oceanografico´ 191, Cidade Universitaria,´ 05508-900 Butanta,˜ SP, Brazil 2Department of Veterinary Disease Biology, University of Copenhagen, Stigbøjlen 7, 1870 Frederiksberg C, Denmark Correspondence should be addressed to Fernando Gomez;´ [email protected] Received 11 July 2015; Accepted 27 August 2015 Academic Editor: Gerardo R. Vasta Copyright © 2015 F. Gomez´ and A. Skovgaard. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Dinoflagellate infections have been reported for different protistan and animal hosts. We report, for the first time, the association between a dinoflagellate parasite and a rotifer host, tentatively Synchaeta sp. (Rotifera), collected from the port of Valencia, NW Mediterranean Sea. The rotifer contained a sporangium with 100–200 thecate dinospores that develop synchronically through palintomic sporogenesis. This undescribed dinoflagellate forms a new and divergent fast-evolved lineage that branches amongthe dinokaryotic dinoflagellates. 1. Introduction form independent lineages with no evident relation to other dinoflagellates [12]. In this study, we describe a new lineage of The alveolates (or Alveolata) are a major lineage of protists an undescribed parasitic dinoflagellate that largely diverged divided into three main phyla: ciliates, apicomplexans, and from other known dinoflagellates. -
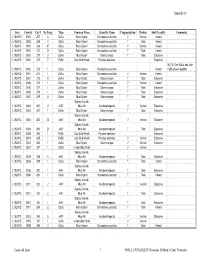
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India.