Chapter 2 Antioxidant Molecules and Redox Cofactors 1 Glutathione A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Sulfite Oxidase R160Q: Identification of the Mutation in a Sulfite Oxidase-Deficient Patient and Expression and Characterization of the Mutant Enzyme
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 6394–6398, May 1998 Medical Sciences Human sulfite oxidase R160Q: Identification of the mutation in a sulfite oxidase-deficient patient and expression and characterization of the mutant enzyme ROBERT M. GARRETT*†,JEAN L. JOHNSON*, TYLER N. GRAF*, ANNETTE FEIGENBAUM‡, AND K. V. RAJAGOPALAN*§ *Department of Biochemistry, Duke University Medical Center, Durham, NC 27710; and ‡Department of Genetics, The Hospital for Sick Children and University of Toronto, 555 University Avenue, Toronto, ON, Canada M5G 1X8 Edited by Irwin Fridovich, Duke University Medical Center, Durham, NC, and approved March 19, 1998 (received for review February 17, 1998) ABSTRACT Sulfite oxidase catalyzes the terminal reac- tion in the degradation of sulfur amino acids. Genetic defi- ciency of sulfite oxidase results in neurological abnormalities and often leads to death at an early age. The mutation in the sulfite oxidase gene responsible for sulfite oxidase deficiency in a 5-year-old girl was identified by sequence analysis of cDNA obtained from fibroblast mRNA to be a guanine to adenine transition at nucleotide 479 resulting in the amino acid substitution of Arg-160 to Gln. Recombinant protein containing the R160Q mutation was expressed in Escherichia coli, purified, and characterized. The mutant protein con- tained its full complement of molybdenum and heme, but exhibited 2% of native activity under standard assay condi- tions. Absorption spectroscopy of the isolated molybdenum domains of native sulfite oxidase and of the R160Q mutant showed significant differences in the 480- and 350-nm absorp- tion bands, suggestive of altered geometry at the molybdenum center. -

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

One Carbon Metabolism and Its Clinical Significance
One carbon metabolism and its clinical significance Dr. Kiran Meena Department of Biochemistry Class 5 : 10-10-2019 (8:00 to 9:00 AM ) Specific Learning Objectives Describe roles of folic acid, cobalamin and S-adenosylmethionine (SAM) in transfer of one carbon units between molecules, and apply their relevance to disease states Describe synthesis of S-adenosylmethionine and its role in methylation reactions Explain how a cobalamin deficiency leads to a secondary folate deficiency Introduction Human body cannot synthesize folic acid Its also called vitamin B9 give rise to tetrahydrofolate (THF), carry one carbon groups ex. Methyl group Intestines releases mostly N5-methy-THF into blood One-carbon (1C) metabolism, mediated by folate cofactor, supports biosynthesis of purines and pyrimidines, aa homeostasis (glycine, serine and methionine) Table 14.4: DM Vasudevan’ s Textbook of Biochemistry for Medical Students 6th edition Enzyme co-factors involved in aa catabolism Involves one of three co-factors: Biotin, Tetrahydrofolate (THF) and S- adenosylmethionine (SAM) These cofactors transfer one-carbon groups in different oxidation states: 1. Biotin transfers carbon in its most oxidized state CO2, it require for catabolism and utilization of branched chain aa • Biotin responsible for carbon dioxide transfer in several carboxylase enzymes Cont-- 2. Tetrahydrofolate (THF) transfers one-carbon groups in intermediate oxidation states and as methyl groups • Tetrahydrobiopterin (BH4, THB) is a cofactor of degradation of phenylalanine • Oxidised form of THF, folate is vitamin for mammals • It converted into THF by DHF reductase 3. S-adenosylmethionine (SAM) transfers methyl groups, most reduced state of carbon THF and SAM imp in aa and nucleotide metabolism SAM used in biosynthesis of creatine, phosphatidylcholine, plasmenylcholine and epinephrine, also methylated DNA, RNA and proteins Enzymes use cobalamin as a cofactor 1. -

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

L-Carnitine, Mecobalamin and Folic Acid Tablets) TRINERVE-LC
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only (L-Carnitine, Mecobalamin and Folic acid Tablets) TRINERVE-LC 1. Name of the medicinal product Trinerve-LC Tablets 2. Qualitative and quantitative composition Each film- coated tablets contains L-Carnitine…………………….500 mg Mecobalamin……………….1500 mcg Folic acid IP…………………..1.5mg 3. Pharmaceutical form Film- coated tablets 4. Clinical particulars 4.1 Therapeutic indications Vitamin and micronutrient supplementation in the management of chronic disease. 4.2 Posology and method of administration For oral administration only. One tablet daily or as directed by physician. 4.3 Contraindications Hypersensitivity to any constituent of the product. 4.4 Special warnings and precautions for use L-Carnitine The safety and efficacy of oral L-Carnitine has not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral L-Carnitine in patients with severely compromised renal function or in ESRD patients on dialysis may result in accumulation of the potentially toxic metabolites, trimethylamine (TMA) and trimethylamine-N-oxide (TMAO), since these metabolites are normally excreted in the urine. Mecobalamin Should be given with caution in patients suffering from folate deficiency. The following warnings and precautions suggested with parent form – vitamin B12 The treatment of vitamin B12 deficiency can unmask the symptoms of polycythemia vera. Megaloblastic anemia is sometimes corrected by treatment with vitamin B12. But this can have very serious side effects. Don’t attempt vitamin B12 therapy without close supervision by healthcare provider. Do not take vitamin B12 if Leber’s disease, a hereditary eye disease. -

Effect of Pyrroloquinoline Quinone Disodium in Female Rats During
Downloaded from British Journal of Nutrition (2019), 121, 818–830 doi:10.1017/S0007114519000047 © The Authors 2019 https://www.cambridge.org/core Effect of pyrroloquinoline quinone disodium in female rats during gestating and lactating on reproductive performance and the intestinal barrier functions in the progeny . IP address: Boru Zhang, Wei Yang, Hongyun Zhang, Shiqi He, Qingwei Meng, Zhihui Chen and Anshan Shan* Institute of Animal Nutrition, Northeast Agricultural University, Harbin 150030, People’s Republic of China 170.106.202.226 (Submitted 26 September 2018 – Final revision received 21 December 2018 – Accepted 28 December 2018 – First published online 28 January 2019) , on Abstract 30 Sep 2021 at 19:42:41 The objective of this study was to investigate the effects of dietary pyrroloquinoline quinone disodium (PQQ·Na2) supplementation on the reproductive performance and intestinal barrier functions of gestating and lactating female Sprague–Dawley (SD) rats and their offspring. Dietary supplementation with PQQ·Na2 increased the number of implanted embryos per litter during gestation and lactation at GD 20 and increased the number of viable fetuses per litter, and the weight of uterine horns with fetuses increased at 1 d of newborn. The mRNA expression levels of catalase (CAT), glutathione peroxidase (GPx2), superoxide dismutase (SOD1), solute carrier family 2 member 1 (Slc2a1) · and solute carrier family 2 member 3 (Slc2a3) in the placenta were increased with dietary PQQ Na2 supplementation. Dietary , subject to the Cambridge Core terms of use, available at supplementation with PQQ·Na2 in gestating and lactating rats increased the CAT, SOD and GPx activities of the jejunal mucosa of weaned rats on PD 21. -
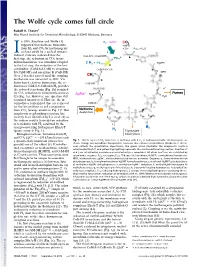
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Mechanism of Reconstitution/Activation of the Soluble PQQ-Dependent Glucose Dehydrogenase from Acinetobacter Calcoaceticus
Mechanism of reconstitution/activation of the soluble PQQ-dependent glucose dehydrogenase from Acinetobacter calcoaceticus: a comprehensive study Claire Stines-Chaumeil, François Mavré, Brice Kauffmann, Nicolas Mano, Benoit Limoges To cite this version: Claire Stines-Chaumeil, François Mavré, Brice Kauffmann, Nicolas Mano, Benoit Limoges. Mecha- nism of reconstitution/activation of the soluble PQQ-dependent glucose dehydrogenase from Acine- tobacter calcoaceticus: a comprehensive study. ACS Omega, ACS Publications, 2020, 10.1021/ac- somega.9b04034. hal-02457126 HAL Id: hal-02457126 https://hal.archives-ouvertes.fr/hal-02457126 Submitted on 27 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Mechanism of reconstitution/activation of the soluble PQQ-dependent glucose dehydrogenase from Acinetobacter calcoaceticus: a comprehensive study Claire Stines-Chaumeil,*,a Francois Mavré,b Brice Kauffmann,c Nicolas Mano,a and Benoit Limoges*,b a CNRS, Université de Bordeaux, CRPP, UMR5031, 115 Avenue Schweitzer, F-33600 Pessac, France. b Université de Paris, Laboratoire d'Electrochimie Moléculaire, UMR 7591, CNRS, F-75013 Paris, France. c CNRS UMS3033, INSERM US001, Université de Bordeaux, IECB, 2, Rue Robert Escarpit, F- 33607 Pessac, France. KEYWORDS: pyrroloquinoline quinone, enzyme reconstitution, enzyme cofactor, apoenzyme, glucose dehydrogenase. -

Prolonging Healthy Aging: Longevity Vitamins and Proteins PERSPECTIVE Bruce N
PERSPECTIVE Prolonging healthy aging: Longevity vitamins and proteins PERSPECTIVE Bruce N. Amesa,1 Edited by Cynthia Kenyon, Calico Labs, San Francisco, CA, and approved September 13, 2018 (received for review May 30, 2018) It is proposed that proteins/enzymes be classified into two classes according to their essentiality for immediate survival/reproduction and their function in long-term health: that is, survival proteins versus longevity proteins. As proposed by the triage theory, a modest deficiency of one of the nutrients/cofactors triggers a built-in rationing mechanism that favors the proteins needed for immediate survival and reproduction (survival proteins) while sacrificing those needed to protect against future damage (longevity proteins). Impairment of the function of longevity proteins results in an insidious acceleration of the risk of diseases associated with aging. I also propose that nutrients required for the function of longevity proteins constitute a class of vitamins that are here named “longevity vitamins.” I suggest that many such nutrients play a dual role for both survival and longevity. The evidence for classifying taurine as a conditional vitamin, and the following 10 compounds as putative longevity vitamins, is reviewed: the fungal antioxidant ergo- thioneine; the bacterial metabolites pyrroloquinoline quinone (PQQ) and queuine; and the plant antioxidant carotenoids lutein, zeaxanthin, lycopene, α-andβ-carotene, β-cryptoxanthin, and the marine carotenoid astaxanthin. Because nutrient deficiencies are highly prevalent in the United States (and elsewhere), appro- priate supplementation and/or an improved diet could reduce much of the consequent risk of chronic disease and premature aging. vitamins | essential minerals | aging | nutrition I propose that an optimal level of many of the known adverse health effects. -

Amino Acid Disorders
471 Review Article on Inborn Errors of Metabolism Page 1 of 10 Amino acid disorders Ermal Aliu1, Shibani Kanungo2, Georgianne L. Arnold1 1Children’s Hospital of Pittsburgh, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; 2Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, MI, USA Contributions: (I) Conception and design: S Kanungo, GL Arnold; (II) Administrative support: S Kanungo; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: E Aliu, GL Arnold; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Georgianne L. Arnold, MD. UPMC Children’s Hospital of Pittsburgh, 4401 Penn Avenue, Suite 1200, Pittsburgh, PA 15224, USA. Email: [email protected]. Abstract: Amino acids serve as key building blocks and as an energy source for cell repair, survival, regeneration and growth. Each amino acid has an amino group, a carboxylic acid, and a unique carbon structure. Human utilize 21 different amino acids; most of these can be synthesized endogenously, but 9 are “essential” in that they must be ingested in the diet. In addition to their role as building blocks of protein, amino acids are key energy source (ketogenic, glucogenic or both), are building blocks of Kreb’s (aka TCA) cycle intermediates and other metabolites, and recycled as needed. A metabolic defect in the metabolism of tyrosine (homogentisic acid oxidase deficiency) historically defined Archibald Garrod as key architect in linking biochemistry, genetics and medicine and creation of the term ‘Inborn Error of Metabolism’ (IEM). The key concept of a single gene defect leading to a single enzyme dysfunction, leading to “intoxication” with a precursor in the metabolic pathway was vital to linking genetics and metabolic disorders and developing screening and treatment approaches as described in other chapters in this issue. -

Molybdenum Cofactor and Sulfite Oxidase Deficiency Jochen Reiss* Institute of Human Genetics, University Medicine Göttingen, Germany
ics: O om pe ol n b A a c t c e e M s s Reiss, Metabolomics (Los Angel) 2016, 6:3 Metabolomics: Open Access DOI: 10.4172/2153-0769.1000184 ISSN: 2153-0769 Research Article Open Access Molybdenum Cofactor and Sulfite Oxidase Deficiency Jochen Reiss* Institute of Human Genetics, University Medicine Göttingen, Germany Abstract A universal molybdenum-containing cofactor is necessary for the activity of all eukaryotic molybdoenzymes. In humans four such enzymes are known: Sulfite oxidase, xanthine oxidoreductase, aldehyde oxidase and a mitochondrial amidoxime reducing component. Of these, sulfite oxidase is the most important and clinically relevant one. Mutations in the genes MOCS1, MOCS2 or GPHN - all encoding cofactor biosynthesis proteins - lead to molybdenum cofactor deficiency type A, B or C, respectively. All three types plus mutations in the SUOX gene responsible for isolated sulfite oxidase deficiency lead to progressive neurological disease which untreated in most cases leads to death in early childhood. Currently, only for type A of the cofactor deficiency an experimental treatment is available. Introduction combination with SUOX deficiency. Elevated xanthine and lowered uric acid concentrations in the urine are used to differentiate this Isolated sulfite oxidase deficiency (MIM#606887) is an autosomal combined form from the isolated SUOX deficiency. Rarely and only in recessive inherited disease caused by mutations in the sulfite oxidase cases of isolated XOR deficiency xanthine stones have been described (SUOX) gene [1]. Sulfite oxidase is localized in the mitochondrial as a cause of renal failure. Otherwise, isolated XOR deficiency often intermembrane space, where it catalyzes the oxidation of sulfite to goes unnoticed.