Placentation in the Anteaters Myrmecophaga
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Digital Reconstruction of the Inner Ear of Leptictidium Auderiense
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Published in "Paläontologische Zeitschrift 90(1): 153–171, 2016" which should be cited to refer to this work. Digital reconstruction of the inner ear of Leptictidium auderiense (Leptictida, Mammalia) and North American leptictids reveals new insight into leptictidan locomotor agility Irina Ruf1,2 • Virginie Volpato1,3 • Kenneth D. Rose4 • Guillaume Billet2,5 • Christian de Muizon5 • Thomas Lehmann1 Abstract Leptictida are basal Paleocene to Oligocene semicircular canals than the leptictids under study. Our eutherians from Europe and North America comprising estimations reveal that Leptictidium was a very agile ani- species with highly specialized postcranial features mal with agility score values (4.6 and 5.5, respectively) including elongated hind limbs. Among them, the Euro- comparable to Macroscelidea and extant bipedal saltatory pean Leptictidium was probably a bipedal runner or jum- placentals. Leptictis and Palaeictops have lower agility per. Because the semicircular canals of the inner ear are scores (3.4 to 4.1), which correspond to the more gener- involved in detecting angular acceleration of the head, their alized types of locomotion (e.g., terrestrial, cursorial) of morphometry can be used as a proxy to elucidate the agility most extant mammals. In contrast, the angular velocity in fossil mammals. Here we provide the first insight into magnitude predicted from semicircular canal angles sup- inner ear anatomy and morphometry of Leptictida based on ports a conflicting pattern of agility among leptictidans, but high-resolution computed tomography of a new specimen the significance of these differences might be challenged of Leptictidium auderiense from the middle Eocene Messel when more is known about intraspecific variation and the Pit (Germany) and specimens of the North American pattern of semicircular canal angles in non-primate Leptictis and Palaeictops. -

Learning About Mammals
Learning About Mammals The mammals (Class Mammalia) includes everything from mice to elephants, bats to whales and, of course, man. The amazing diversity of mammals is what has allowed them to live in any habitat from desert to arctic to the deep ocean. They live in trees, they live on the ground, they live underground, and in caves. Some are active during the day (diurnal), while some are active at night (nocturnal) and some are just active at dawn and dusk (crepuscular). They live alone (solitary) or in great herds (gregarious). They mate for life (monogamous) or form harems (polygamous). They eat meat (carnivores), they eat plants (herbivores) and they eat both (omnivores). They fill every niche imaginable. Mammals come in all shapes and sizes from the tiny pygmy shrew, weighing 1/10 of an ounce (2.8 grams), to the blue whale, weighing more than 300,000 pounds! They have a huge variation in life span from a small rodent living one year to an elephant living 70 years. Generally, the bigger the mammal, the longer the life span, except for bats, which are as small as rodents, but can live for up to 20 years. Though huge variation exists in mammals, there are a few physical traits that unite them. 1) Mammals are covered with body hair (fur). Though marine mammals, like dolphins and whales, have traded the benefits of body hair for better aerodynamics for traveling in water, they do still have some bristly hair on their faces (and embryonically - before birth). Hair is important for keeping mammals warm in cold climates, protecting them from sunburn and scratches, and used to warn off others, like when a dog raises the hair on its neck. -

New Large Leptictid Insectivore from the Late Paleogene of South Dakota, USA
New large leptictid insectivore from the Late Paleogene of South Dakota, USA TJ MEEHAN and LARRY D. MARTIN Meehan, T.J. and Martin, L.D. 2012. New large leptictid insectivore from the Late Paleogene of South Dakota, USA. Acta Palaeontologica Polonica 57 (3): 509–518. From a skull and mandible, we describe a new genus and species of a primitive insectivore (Mammalia: Insectivora: Leptictida: Leptictidae). Its large body size and higher−crowned teeth indicate a different feeding ecology from other leptictid insectivores. With evidence of some heavy, flat wear on the molariform teeth, its shift in diet was likely to greater herbivory. Unlike the narrow snout of Blacktops, this new leptictid retains a broad snout, suggesting that small verte− brates were still important dietary components. The specimen was collected from the floodplain deposits of the lower or middle White River Group of South Dakota, which represent the latest Eocene to earliest Oligocene (Chadronian and Orellan North American Land Mammal “Ages”). Key words: Mammalia, Leptictidae, Leptictis, Megaleptictis, Eocene, Oligocene, White River Group, South Dakota, North America. TJ Meehan [[email protected]], Research Associate, Section of Vertebrate Paleontology, Carnegie Museum of Natural History, 4400 Forbes Avenue, Pittsburgh, PA 15213, USA; Larry D. Martin [[email protected]], Division of Vertebrate Paleontology, Natural History Museum and Biodiversity Re− search Center, University of Kansas, Lawrence, KS 66045, USA. Received 4 April 2011, accepted 25 July 2011, available online 17 August 2011. Introduction molariform teeth. A fossa in this region at least suggests in− creased snout mobility, but no definitive anatomical argument Leptictida is a primitive order of placental, insectivorous has been made to support a highly mobile cartilaginous snout mammals convergent to extant sengis or elephant “shrews” tip, as in sengis. -

Eutheria (Placental Mammals)
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction have supernumerary teeth (e.g. some whales, armadillos, Eutheria (or Placentalia) is the most taxonomically diverse etc.), in extant placentals the number of teeth is at most of three branches or clades of mammals, the other two three upper and lower incisors, one upper and lower being Metatheria (or Marsupialia) and Prototheria (or canine, four upper and lower premolars, and three upper Monotremata). When named by Gill in 1872, Eutheria and lower molars. Except for one fewer upper molar, a included both marsupials and placentals. It was Huxley in domestic dog retains this pattern. Compared to reptiles, 1880 that recognized Eutheria basically as used today to mammals have fewer skull bones through fusion and loss, include only placentals. McKenna and Bell in their although bones are variously emphasized in each of the Classification of Mammals, published in 1997, chose to three major mammalian taxa. use Placentalia rather than Eutheria to avoid the confusion Physiologically, mammals are all endotherms of varying of what taxa should be included in Eutheria. Others such as degrees of efficiency. They are also homeothermic with a Rougier have used Eutheria and Placentalia in the sense relatively high resting temperature. These characteristics used here. Placentalia includes all extant placentals and are also found in birds, but because of anatomical their most recent common ancestor. -
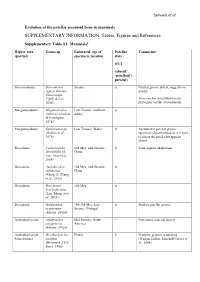
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Highly Derived Eutherian Mammals from the Earliest Cretaceous of Southern Britain
Highly derived eutherian mammals from the earliest Cretaceous of southern Britain STEVEN C. SWEETMAN, GRANT SMITH, and DAVID M. MARTILL Sweetman, S.C., Smith, G., and Martill, D.M. 201X. Highly derived eutherian mammals from the earliest Cretaceous of southern Britain. Acta Palaeontologica Polonica XX (X): xxx–xxx. Eutherian mammals (Placentalia and all mammals phylogenetically closer to placentals than to marsupials) comprise the vast majority of extant Mammalia. Among these there is a phenomenal range of forms and sizes, but the origins of crown group placentals are obscure. They lie within the generally tiny mammals of the Mesozoic, represented for the most part by isolated teeth and jaws, and there is strongly conflicting evidence from phenomic and molecular data as to the date of origin of both Eutheria and Placentalia. The oldest purported eutherians are Juramaia from the Upper Jurassic of China, and Eomaia and Acristatherium from the Lower Cretaceous, also of China. Based on dental characters and analyses of other morphological and molecular data, doubt has recently been cast on the eutherian affinities of the Chinese taxa and consequently on the date of emergence of Eutheria. Until now, the only tribosphenic mammal recorded from the earliest Cretaceous (Berriasian) Purbeck Group of Britain was the stem tribosphenidan Tribactonodon. Here we document two new tribosphenic mammals from the Purbeck Group, Durlstotherium gen. nov. and Durlstodon gen. nov., showing highly derived eutherian molar characters that support the early emergence of this clade, prior to the Cretaceous. Key words: Mammalia, Eutheria, dentition, Early Cretaceous, Purbeck Group, Britain. Steven C. Sweetman [[email protected]], Grant Smith [[email protected]], and David M. -

A Radiation of Arboreal Basal Eutherian Mammals Beginning in the Late Cretaceous of India
A radiation of arboreal basal eutherian mammals beginning in the Late Cretaceous of India Anjali Goswamia,b,1, Guntupalli V. R. Prasadc, Paul Upchurchb, Doug M. Boyerd, Erik R. Seifferte, Omkar Vermaf, Emmanuel Gheerbrantg, and John J. Flynnh aDepartment of Genetics, Evolution, and Environment, bDepartment of Earth Sciences, University College London, London WC1E 6BT, United Kingdom; cDepartment of Geology, Centre for Advanced Studies, University of Delhi, Delhi 110 007, India; dDepartment of Anthropology and Archaeology, Brooklyn College, City University of New York, Brooklyn, NY 11210; eDepartment of Anatomical Sciences, Stony Brook University, Stony Brook, NY 11794-8081; fSchool of Sciences, Indira Gandhi National Open University, New Delhi 110 068, India; gUnité Mixte de Recherche 7207 du Centre National de la Recherche Scientifique (CR2P), Département Histoire de la Terre, Muséum National d’Histoire Naturelle, 75005 Paris, France; and hDivision of Paleontology and Richard Gilder Graduate School, American Museum of Natural History, New York, NY 10024 Edited* by Elwyn L. Simons, Duke University, Durham, NC, and approved August 15, 2011 (received for review June 6, 2011) India’s Late Cretaceous fossil mammals include the only undis- cluding placentals and their stem relatives) are known from the puted pre-Tertiary Gondwanan eutherians, such as Deccanolestes. Late Cretaceous of Laurasia (North America, Europe, and Asia) Recent studies have suggested a relationship between Deccano- (9), and although a few have been suggested as possible pla- lestes and African and European Paleocene adapisoriculids, which centals [e.g., Protungulatum (10)], none are unequivocally sup- have been variably identified as stem euarchontans, stem pri- ported as a Cretaceous placental mammal (2). -

Morphology and Physiology of the Eutheria
FAUNA of AUSTRALIA 34. MORPHOLOGY AND PHYSIOLOGY OF THE EUTHERIA M.M. BRYDEN 1 34. MORPHOLOGY AND PHYSIOLOGY OF THE EUTHERIA 2 34. MORPHOLOGY AND PHYSIOLOGY OF THE EUTHERIA TERMINOLOGY The language of biology is vital in imparting knowledge and exchanging ideas. The language must be precise, because confusion can (and does) arise if multiple terms are used to describe a structure or physiological process. Veterinary anatomists have attempted to overcome misconceptions in biology due to confusion in terms by developing a standard set of names for structures (Nomina Anatomica Veterinaria and Nomina Histologica 1983). Adoption of appropriate terms from that source for the Eutheria generally would be beneficial to biologists, and at the risk of offending those who fear some of their favourite terms are threatened, I shall follow the standard terms of the Nomina, or their English equivalents, in this chapter. EXTERNAL BODY FORM Eutheria in Australia range in size from the smallest bats, with body weight of 5 g or less, to the large whales, with body weights exceeding 100 tonnes. Although bearing the basic characters that distinguish mammals generally, namely the presence of hair and mammary glands, the external form of Australian Eutheria is very variable (Fig. 34.1). This is related partly to the environment in which they live, which varies from totally aquatic to arid desert. Small rodents have a dense pelage, whereas the totally aquatic mammals (dugongs and whales) have little or no hair on the general body surface. Mammals that need to conserve body heat, notably the aquatic mammals, have fusiform bodies and small appendages, presenting to the environment a relatively small body surface area per unit weight. -

Phylogenetics
background sheet Phylogenetics Taxonomy: the classification of life To make sense of biodiversity scientists have attempted to organise, or group, life forms. In the eighteenth century, Carl Linnaeus developed a system of classification that organised the living world into ranks, and introduced binomial nomenclature, or scientific names. Linnaean classification is hierarchical and groups organisms according to similarities and differences. Traditional, or Linnaean taxonomy, transformed the way in which organisms were named, described and ranked. But these ideas were developed long before there was any scientific understanding of the evolution of life. In 1859, publication of Charles Darwin’s work provided a new theoretical framework that saw a shift in focus of taxonomy. The new taxonomy As our understanding of relationships between organisms has changed, so has the focus of taxonomy. Modern taxonomy strives to reflect evolutionary history of organisms, by classifying them according to evolutionary relationships. This approach to taxonomy, known as phylogenetic systematics, uses a basic unit of classification called ‘clade’. Clade — a group consisting of an ancestral form and all living and extinct descendants of that ancestor. Members of a clade share a common ancestor to the exclusion of all other organisms. The Linnaean classification system is still used in modern taxonomy, and in many instances phylogenetic analyses reflect Linnaean groupings. In some cases Linnaean groupings have been modified to reflect new understanding of evolutionary relationships by the addition of extra ranks, such as superorder. Molecular evidence for evolution Prior to the 1970s, evidence for evolutionary relationships between organisms was based on similarities and differences of physical characteristics: fossil, anatomical and embryological features. -

Eutheria (Placental Mammals) Thought of As More Primitive
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction doi: 10.1038/npg.els.0004123 Eutheria (or Placentalia) is the most taxonomically diverse each. Except for placentals that have supernumerary teeth of three branches or clades of mammals, the other two (e.g. some whales, armadillos, etc.), in extant placentals, the being Metatheria (or Marsupialia) and Prototheria (or number of teeth is at most three upper and lower incisors, Monotremata). When named by Gill in 1872, Eutheria in- one upper and lower canine, four upper and lower premo- cluded both marsupials and placentals. It was Huxley in lars and three upper and lower molars. Pigs retain this pat- 1880 who recognized Eutheria basically as used today to tern, and except for one fewer upper molar, a domestic dog include only placentals. McKenna and Bell in their Clas- does as well. Compared to reptiles, mammals have fewer sification of Mammals published in 1997, chose to use Pla- skull bones through fusion and loss, although bones are centalia rather than Eutheria to avoid the confusion of variously emphasized in each of the three major mammalian what taxa should be included in Eutheria. Others such as taxa. See also: Digestive system of mammals; Ingestion in Rougier have used Eutheria and Placentalia in the sense mammals; Mesozoic mammals; Reptilia (reptiles) used here. Placentalia includes all extant placentals and Physiologically, mammals are all endotherms with var- their most recent common ancestor. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL. 29. NO. 10. PP. 259-289 November 30. 1995 EVOLUTION OF CORYPHODON (MAMMALIA, PANTODONTA) IN THE LATE PALEOCENE AND EARLY EOCENE OF NORTHWESTERN WYOMING BY MARK D. UHEN AND PHILIP D. GINGERICH MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTFUBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Philip D. Gingerich, Director This series of contributions from the Museum of Paleontology is a medium for publication of papers based chiefly on collections in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals on request. A list of the separate issues may also be obtained by request. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan 48109-1079. VOLS. 2-29. Parts of volumes may be obtained if available. Price lists are available upon inquiry. EVOLUTION OF CORYPHODON (MAMMALIA, PANTODONTA) IN THE LATE PALEOCENE AND EARLY EOCENE OF NORTHWESTERN WYOMING MARK D. UHEN AND PHILIP D. GINGERICH Abstract-Six species of Coryphodon are recognized from the late Paleocene and early Eocene of the Bighorn and Clarks Fork Basins in northwestern Wyoming. These are, in order of temporal appearance: Coryphodon proterus, Coryphodon eocaenus, Coryphodon radians, Coryphodon armatus, and Coryphodon lobatus. C. proterus is the only species known from the Clarkforkian land-mammal age. The remaining taxa are restricted to some portion of the Wasatchian land-mammal age. The Coryphodon lineage including C. -

The Earliest Known Eutherian Mammal
articles The earliest known eutherian mammal Qiang Ji*, Zhe-Xi Luo†, Chong-Xi Yuan*, John R. Wible†, Jian-Ping Zhang‡ & Justin A. Georgi† * Chinese Academy of Geological Sciences, Beijing 100037, China † Carnegie Museum of Natural History, 4400 Forbes Avenue, Pittsburgh, Pennsylvania 15213, USA ‡ Geoscience University of China, Beijing 100083, China ........................................................................................................................................................................................................................... The skeleton of a eutherian (placental) mammal has been discovered from the Lower Cretaceous Yixian Formation of northeastern China. We estimate its age to be about 125 million years (Myr), extending the date of the oldest eutherian records with skull and skeleton by about 40–50 Myr. Our analyses place the new fossil at the root of the eutherian tree and among the four other known Early Cretaceous eutherians, and suggest an earlier and greater diversification of stem eutherians that occurred well before the molecular estimate for the diversification of extant placental superorders (104–64 Myr). The new eutherian has limb and foot features that are known only from scansorial (climbing) and arboreal (tree-living) extant mammals, in contrast to the terrestrial or cursorial (running) features of other Cretaceous eutherians. This suggests that the earliest eutherian lineages developed different locomotory adaptations, facilitating their spread to diverse niches in the Cretaceous. Placental