Winter Ecology of Brown Trout in Groundwater Buffered Streams a DISSERTATION SUBMITTED to the FACUL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zumbro River Comprehensive Watershed Management Plan
Greater Zumbro River Watershed Comprehensive Watershed Management Plan Waterside Chats Summary Summary The Greater Zumbro River Watershed Partnership hosted “Waterside Chats” in three communities throughout the watershed in October and November 2019. Waterside Chats were held on October 24th, 2019 at the Zumbro Valley Recreation Club in Mantorville, November 7th, 2019 at the Community Center in Mazeppa and November 14th, 2019 at the Sportsman’s Club in Lake City. The public was asked to attend the Waterside Chats to learn about the issues that had been identified by local partners and to provide feedback with their local knowledge of the watershed. Each Waterside Chat began with an overview presentation by the local SWCD or County Staff which included a summary of the One Watershed, One Plan program and plan development process, a summary of what has been accomplished, and information on how the public can participate. Following the overview, Barr Engineering summarized the priority resources and issues that had been identified in local and state plans, studies, reports, state agency feedback, and resident surveys. Initial results of the prioritization of these issues identified by a survey of watershed residents and ranked by the policy committee, planning workgroup and technical advisory group was also shared to aid in the table conversations (see figure 1 below). Following the presentation, attendees were broken into small groups. Each small group discussed a series of questions to provide their input and feedback on the list of priority issues to be addressed in the 10-year scope of the plan. Comments were captured by a facilitator from the planning partnership, summarized, and reported out to the large group. -

FISHING GUIDE Lake Pepin Offers Fantastic Small Mouth Bass 63 Fishing in All Seasons
Mpls./St. 68 miles P Bass Fishing aul FISHING GUIDE Lake Pepin offers fantastic Small Mouth Bass 63 fishing in all seasons. Fish the many rip rap For Lake Pepin Along the Mississippi River areas and points with plastics, crankbaits and spinners. Please practice catch and 35 release on these fish. The lakes true potential can only be realized with voluntary release of Bay City these fish. Pike Fishing Fish the many shoreline weed beds in all 1 seasons. During the summer target the Red mouths of the many cold water tributaries 61 WISCONSIN that enter the lake. Trophy pike often lay in Wing MINNESOT Rush River the cooler waters that these streams provide. 63 2 White Bass Fishing Fish the many points of the lake for whites. A Maiden Rock Watch for feeding gulls as an indicator for surface feeding white bass. 1 Mile Point-No-Point 4 Lake City Area Public Landings 1 Florence Township Public Landing: Frontenac Old Frontenac, MN SP 3 Concrete slab landing with limited parking. 5 Pine Creek 1 2 Hok-Si-La Park Landing: Lake City, MN Frontenac Concrete slab landing, docks, and picnic Little Pine Creek area with ample parking. 6 3 Roschen Park Landing: Wells Creek Lake City, MN Concrete slab landing, docks, fish cleaning facility and picnic area with ample parking. 61 7 Lake City also offers a full service 8 marina, fee landing and docking for Hok-Si-La Park 35 small and large boats with daily and 2 Stockholm overnight rates. Visit www.LakeCityMN.org for more 9 Bogus Point information on fishing amenities in the Lake City e Lake City area. -

Executive Summary
Executive Summary During its fifth full year of operations in fiscal year 2007-08, the N.C. Ecosystem Enhancement Program (EEP) took on new challenges and continued to register fresh successes in meeting its goals of improving North Carolina’s environment while facilitating responsible economic growth for the state and its residents. EEP continued to collaborate with federal, state and local governments, contractors and willing landowners to provide goods and services, basing its work on a solid foundation of watershed plan- ning that goes beyond mere environmental permitting and compliance. The initiative also extended its record of carrying out its mission without a single transportation-project delay due to the lack of mitigation, helping to move forward more than $4.8 billion in transportation-infrastructure im- provements since becoming operational in 2003. Key developments in FY 2007-08 included: • Responding to changes to federal rules guiding aquatic-resource mitigation. Under the leadership of the Governor’s office, N.C. Department of Environment and Natural Resources (NCDENR) and EEP petitioned the U.S. Office of Management and Budget, the U.S. Environ- mental Protection Agency and U.S. Army Corps of Engineers (USACE) in late 2007 to promote in- lieu fee programs such as EEP as a method to providing third-party mitigation for public- and pri- vate-sector development. The new rule, which became effective in June of 2008, gave recognition to EEP’s unique national status and maintained ILFs as a viable option. The rule will require EEP to make operational adjustments and the details of these changes are being evaluated. -

7050.0470 CLASSIFICATIONS for SURFACE WATERS in MAJOR DRAINAGE BASINS. Subpart 1. Lake Superior Basin. the Water Use Classifica
1 REVISOR 7050.0470 7050.0470 CLASSIFICATIONS FOR SURFACE WATERS IN MAJOR DRAINAGE BASINS. Subpart 1. Lake Superior basin. The water use classifications for the listed waters in the Lake Superior basin are as identified in items A to D. See parts 7050.0425 and 7050.0430 for the classifications of waters not listed. A. Streams: (1) Ahlenius Creek, (T.53, R.14, S.9, 10): 1B, 2A, 3B; (2) Amenda Creek, (T.59, R.5, S.19, 20, 29, 30, 31; T.59, R.6, S.36): 1B, 2A, 3B; (3) Amity Creek, (T.50, R.13, S.5, 6; T.50, R.14, S.1; T.51, R.13, S.31, 32; T.51, R.14, S.26, 27, 28, 35, 36): 1B, 2A, 3B; (4) Amity Creek, East Branch (T.51, R.13, S.30, 31; T.51, R.14, S.13, 14, 15, 22, 24, 25, 36): 1B, 2A, 3B; (5) Anderson Creek, Carlton County, (T.46, R.17, S.11, 14, 15, 22, 26, 27): 1B, 2A, 3B; (6) Anderson Creek, St. Louis County, (T.49, R.15, S.16, 17, 18; T.49, R.16, S.12, 13): 1B, 2A, 3B; (7) Artichoke Creek, (T.52, R.17, S.7, 17, 18): 1B, 2A, 3B; (8) Assinika Creek, (T.63, R.1E, S.1; T.63, R.2E, S.7, 8, 16, 17, 21; T.64, R.1E, S.36; T.64, R.2E, S.31): 1B, 2A, 3B; (9) Bally Creek, (T.61, R.1W, S.3, 4, 5, 6, 7, 8, 9, 10, 11; T.61, R.2W, S.12): 1B, 2A, 3B; (10) Baptism River, East Branch, (T.57, R.6, S.6; T.57, R.7, S.1, 2, 3, 9, 10, 11, 12, 16, 17, 20; T.58, R.6, S.30, 31; T.58, R.7, S.13, 17, 19, 20, 21, 22, 23, 24, 25, 26, 29, 30, 36; T.58, R.8, S.22, 23, 24, 25, 26): 1B, 2A, 3B; (11) Baptism River, Main Branch, (T.56, R.7, S.3, 4, 5, 9, 10, 14, 15; T.57, R.7, S.20, 27, 28, 29, 33, 34): 1B, 2A, 3B; (12) Baptism River, West Branch, (T.57, R.7, S.7, 17, 18, 20; T.57, R.8, S.1, 2, 12; T.58, R.8, S.2, 3, 4, 9, 10, 11, 15, 16, 20, 21, 22, 28, 33, 34, 35, 36; T.59, R.8, S. -

(HSA) Generated Sunday January 24 2021
NCRFC forecast points by Hydrologic Service Area (HSA) Generated Sunday August 15 2021 FCST TYPE LEGEND: B - Benchmark Tributary (Apr-Oct) H - Headwater/WFO Site Specific (FFH guidance) D - Daily morning fcst during drought only I - Inflow fcst E - Evening daily fcst M - Morning daily fcst F - Flood event-only fcst W - Long Range Water Supply FS - Flood Stage RFC FIS - Forecast Issuance Stage WFO Action - WFO Action Stage MINOR - Minor flooding begins MOD - Moderate flooding begins MAJOR - Major Flooding Begins # - Not currently an active realtime USGS Surface Water Station. *** ABR - Aberdeen, SD *** RFC WFO FCST NWSLI USGS ID Group Station Name River Name FS FIS Action MINOR MOD MAJOR LAT (N) LON (W) TYPE ----- ---------- ----- ------------ ---------- -- --- ------ ----- --- ----- ------- ------- ---- PVRS2 05290000 MIN Peever 6NE Little Minnesota River 17.0 16.0 16.0 17.0 22.0 24.0 45.6161 96.8753 FH BSLM5 MIN Ortonville - Big Stone Lk Minnesota River 971.5 970.5 970.5 971.5 973.0 975.0 45.3092 96.4681 F *** APX - N. Central Lower Michigan, MI *** RFC WFO FCST NWSLI USGS ID Group Station Name River Name FS FIS Action MINOR MOD MAJOR LAT (N) LON (W) TYPE ----- ---------- ----- ------------ ---------- -- --- ------ ----- --- ----- ------- ------- ---- SHRM4 04124000 NLM Sherman 1N Manistee River 15.0 14.0 14.0 15.0 16.0 17.0 44.4364 85.6986 MF MYFM4 04126970 NLM Mayfield 5NE - Brown Brdg Rd Boardman River 7.0 5.0 5.0 7.0 9.0 11.0 44.6567 85.4367 FH RDOM4 04136000 NLM Red Oak 3S Au Sable River 7.0 6.0 6.0 7.0 8.0 9.0 44.6769 84.2925 MF -

2011 Trout Angling Opportunities in Southern and Central Minnesota
2011 Trout Angling Opportunities in Southern and Central Minnesota The Minnesota Department of Natural Resources published this set of maps to help anglers locate trout fishing opportunities in southeast, southwest and central Minnesota. These maps use different colors to show angling regulations for 2011 and identify areas with public fishing in state parks, state forests, wildlife management areas, and streamside-fishing easements. Updated maps will be available on the DNR web site at: www.mndnr.gov/fishing/trout_streams Turn In Poachers 1-(800) 652-9093 (Dial #TIP for Cingular, Midwest Wireless, Unicel and Verizon cell phone customers) This document is made available electronically by the Minnesota Legislative Reference Library as part of an ongoing digital archiving project. http://www.leg.state.mn.us/lrl/lrl.asp Southeast and Central Fisheries Crystal Springs State Fish Hatchery 14674 County # 112 Offices and Hatcheries Altura, MN 55910 507-796-6691 [email protected] Regional Fisheries Office 1200 Warner Road Lanesboro State Fish Hatchery St. Paul, MN 55155 23789 Grosbeak Road 651-259-5769 Lanesboro, MN 55949 507-467-3771 Hinckley Area Fisheries Office (311) [email protected] P.O. Box 398 306 Power Avenue North Peterson State Fish Hatchery Hinckley, MN 55037 28376 County Road 25 South 320-384-7721 Peterson, MN 55962 [email protected] 507-875-2625 [email protected] Little Falls Area Fisheries Office (312) 16543 Haven Road Southwest Fisheries Offices Little Falls, MN 56345 320-616-2450 ext 225 [email protected] Regional Fisheries Office 261 Highway 15 South East Metro Area Fisheries Office (313) New Ulm, MN 56073-8915 1200 Warner Road 507-359-6088 St. -

Lakes 0 ) Reams
fi ° ckle' via nove. ey • MINNESOTA lakes 08) reams 'Published by The Bureau of Information Minnesota Department of Conservation Room 633 State Office Building Saint Paul eXinnesota SOME OF MINNESOTA'S GAME FISH NORTHERN PIKE WALLEYE Esox lucius Stizostedion vitreum BROOK TROUT RAINBOW TROUT Salvelinus fontinalis SoImo gairdnerii MUSKELLUNGE LARGEMOUTH BASS Esox masquinongy masquinongy Huro salmoides BLACK CRAPPIE BLUEGILL Pomoxis nigro-maculatus Lepomis macrochirus (cuts through courtesy of James Heddon's Sons Dowagiac, Michigan) PUMPKINSEED Lepomis gibbosus MINNESOTA fish Lakes and Streams Published by the BUREAU OF INFORMATION, DEPARTMENT OF CONSERVATION Room 633 — State Office Saint Paul 1 Minnesota RECORD FISH CATCHES BY ANGLING IN MINNESOTA Black crappie ......... 5 lb. Muskellunge ......... 50 lb. Bluegill ................ 2 lb. 13 oz. Northern pike ....... 41 lb. Brook trout ........... 4 lb. 3 oz. Rainbow trout ....... 13 lb. 2 oz. Lake trout ............ 42 lb. Smallmouth bass 8 lb. Largemouth bass ....8 lb. 15 oz. Walleye ................ 16 lb. 8 oz. 1 FISHING IN MINNESOTA By Dr. George A. Selke, Commissioner, Minnesota Dept. of Conservation Minnesota needs no other press agent than the invitation and promise of 10,000 lakes-11,007 lakes to be exact, 25,000 miles of streams and water- ways, and headwaters of the Mississippi. We offer you the matchless climate of Minnesota summertime — the hospitality of the good people of our state—churches for your Sunday wor- ship during your sojourn with us—and hospitals of world renown for your every medical need. We offer you the scenic wonders of our State Parks—the breathtaking beauty of the North Shore Drive—the canoe trails of the Roadless Wilder- ness area—incomparable Lake Superior and Lake of the Woods—the tum- bling cascades of the Temperance and the Baptism—the much visited White- water area—the charming peace and quiet of southern Minnesota lakes— and the far-reaches of rolling prairies and fertile acres stretching into the pine forests and iron ranges of the North. -
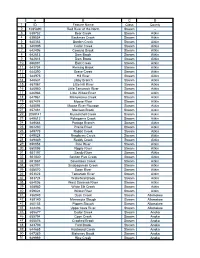
List of MN Rivers and Streams
A B C D 1 ID Feature Name Class County 2 1035890 Red River of the North Stream - 3 639752 Bear Creek Stream Aitkin 4 639854 Beckman Creek Stream Aitkin 5 640383 Borden Creek Stream Aitkin 6 640995 Cedar Creek Stream Aitkin 7 642406 Cowans Brook Stream Aitkin 8 642613 Dam Brook Stream Aitkin 9 642614 Dam Brook Stream Aitkin 10 656091 East Creek Stream Aitkin 11 643734 Fleming Brook Stream Aitkin 12 644390 Grave Creek Stream Aitkin 13 644975 Hill River Stream Aitkin 14 646631 Libby Branch Stream Aitkin 15 657067 Little Hill River Stream Aitkin 16 646950 Little Tamarack River Stream Aitkin 17 646966 Little Willow River Stream Aitkin 18 647961 Minnewawa Creek Stream Aitkin 19 657474 Moose River Stream Aitkin 20 648094 Moose River Flowage Stream Aitkin 21 657481 Morrison Brook Stream Aitkin 22 2059141 Musselshell Creek Stream Aitkin 23 649612 Pokegama Creek Stream Aitkin 24 649664 Portage Branch Stream Aitkin 25 662230 Prairie River Stream Aitkin 26 649778 Rabbit Creek Stream Aitkin 27 649828 Raspberry Creek Stream Aitkin 28 649889 Reddy Creek Stream Aitkin 29 650053 Rice River Stream Aitkin 30 650096 Ripple River Stream Aitkin 31 651197 Sandy River Stream Aitkin 32 651830 Section Five Creek Stream Aitkin 33 651867 Seventeen Creek Stream Aitkin 34 652091 Sisabagamah Creek Stream Aitkin 35 658570 Swan River Stream Aitkin 36 653023 Tamarack River Stream Aitkin 37 653724 Wakefield Brook Stream Aitkin 38 654006 West Savanna River Stream Aitkin 39 658982 White Elk Creek Stream Aitkin 40 659024 Willow River Stream Aitkin 41 456043 Duck Creek Stream -

Surface Water Supply of the United States 1909 Part V
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR WATER-SUPPLY PAPER 265 SURFACE WATER SUPPLY OF THE UNITED STATES 1909 PART V. HUDSON BAY AND UPPER MISSISSIPPI RIVER BASINS PREPARED UNDER THE DIRECTION OF M. 0. LEIGHTON BY ROBERT FOLLANSBEE, A. H. HORTON AND R. H. BOLSTER WASHINGTON GOVERNMENT PRINTING OFFICE 1911 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR WATER-SUPPLY PAPER 265 SURFACE WATER SUPPLY OF THE UNITED STATES 1909 PART V. HUDSON BAY AND UPPER MISSISSIPPI R1YER BASINS PREPARED UNDER THE DIRECTION OF M. 0. LEIGHTON BY ROBERT FOLLANSBEE, A. H. HORTON AND R. H. BOLSTER WASHINGTON GOVERNMENT PRINTING OFFICE 1911 CONTENTS. Introduction............................................................. 7 Authority for investigations............................................. 7 Scope of investigations.................................................. 8 Purposes of the work.................................................. 9 Publications.......................................................... 10 Definition of terms..................................................... 13 Convenient equivalents................................................ 14 Explanation of tables................................................... 15 Field methods of measuring stream flow................................. 16 Office methods of computing and studying discharge and run-off.......... 22 Accuracy and reliability of field data and comparative results............. 26 Use of the -

Locations of Streamflow-Gaging Stations, Crest Gages, Regulated Stations, and Flood-Frequency Areas in Wisconsin and Adjacent States
U.S. Department of the Interior Prepared in cooperation with the Scientific Investigations Report 2016−5140 U.S. Geological Survey Wisconsin Department of Transportation Plate 1 of 1 94° 92° 90° 88° 86° ge River id Baptism River tr r West Two River a P East Swan River L A K E S U r P E ive R I et R O qu R lo C Gooseberry River ce River efa it h W 47° r Rive er quet iv lo R C n Misery River a w S Louis R Saint iver O 04026200 n r to Otter River ive Sand River n i R a er p g v ip o i s n R is Iron River R iss n i o M Bois Brule River ve Mulligan Creek r e g Midway River r tu S Bardon Creek Bardon Sioux River 04024430 04026300 Nem 04024400 r 04025200 040263205 Trout Creek adji ive Rock River River R B 04029990 a M d M id 04025500 North Fish Creek R d 040263491 Beartrap Creek i o Sucker Creek le v n icon 04027500 04029700 t B mn e Pot re r A r ato R a a iv l n c 04027000 e 04028000 R h iver r E o R er F i ng yl o v sc e e r T r an a k 04029000 r Fe a s n b M ce a R R iv Long Lake Branch i er 04026700 ve Mississippi River 04027200 r MICHIGAN r ive Net River 04026450 e R rtl r 04026400 u e tagatic 05357390 T v To River 05331833 i P R r ai e wish R n v nito iv t R e i a er l R i t M v t 05333100 a Trout River e e Sand Creek 1 w Bear River 05333500 pe r K 05357500 05357225 ip 05390140 E h sc C 05357360 a r rk e R n ive o Brul ive a on R F 05358000 r b kag st 05357335 05357245 a e a R 05332500 am E 05357215 04059900 N 04060993 04063000 iv W 05358100 Deerskin River F er e 05336000 r o s B e 04063640 r t C e iv 05392150 v r 04062011 d B l an Ri -

Driftless Area Land Cover and Land Use
Driftless Area Land Cover and Land Use Bruce Vondraceka,1 aDepartment of Fisheries, Wildlife, and Conservation Biology, University of Minnesota, St. Paul, Minnesota, USA This manuscript was compiled on February 5, 2019 1. Settlement of the Driftless Area by Europeans between 1850 and by Native Americans, likely to perpetuate bison and other and 1935 altered the landscape through intensive agriculture and re- large animals, e.g. elk Cervus canadensis. Forests were north- moval of forest cover, which resulted in significant sediment delivery ern deciduous hardwoods. Forests on north-facing slopes were to streams. sugar maple Acer saccharum, beech Fagus spp., and basswood 2. Replacement of forest and vegetative cover with row crops or con- Tilia americana, whereas bur oaks Quercus macrocarpa were tinuously grazed pastures altered flow regimes in streams because found on sunny slopes. Many steep southern and western of reduced ability of the catchment to absorb precipitation. slopes were treeless. Vegetation on floodplains and terraces 3. Beginning in 1935, after instituting the first watershed-scale soil were varied with trees in some areas and grasslands in other and water conservation demonstration project in the United States in areas. Trees were maples A. spp., birch Populus spp., and elm the Coon Creek watershed, cropping systems and land management Ulmus spp.. Streambanks could often be lined with trees, even changed in the Driftless Area, which led to reduced sediment losses when the adjacent areas were grassland. However, sketches by from the landscape. early visitors indicate no more than 20% of the streambanks were lined with trees. Floodplains were characterized as hav- Land Cover and Use | Agriculture | Water Quality | Stream Habitat | ing a dark well-developed non-stratified soil with little vertical Biotic Integrity accretion. -

Implementation Plan for the Lower Vermillion River Turbidity Total Maximum Daily Load (TMDL)
MISSISSIPPI MAKEOVER A Plan for Restoration in Spring Lake (Pool 2), Mississippi River – Pool 3 & The Lower Vermillion River An Implementation Plan for the Lower Vermillion River Turbidity Total Maximum Daily Load (TMDL) A Scoping Plan for Implementation in Spring Lake, Pool 2 and Pool 3 of the South Metro Mississippi River TSS TMDL June 2011 Prepared for the Minnesota Pollution Control Agency by Dakota County and the Dakota County Soil and Water Conservation District 0 | Mississippi Makeover Implementation P l a n – J u n e 2011 Contents 1.0 Executive Summary ................................................................................................................................. 2 1.1 Acknowledgements ............................................................................................................................. 3 1.2 List of Acronyms .................................................................................................................................. 4 2.0 Mississippi Makeover Project ................................................................................................................. 5 2.1 Project Area ........................................................................................................................................ 5 2.2 Development of Indicators by Citizen Advisory Group ....................................................................... 8 2.3 Input from Technical Experts .............................................................................................................