Chemical Profile and Antimicrobial Activity of the Fungus-Growing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Termites in a Structure
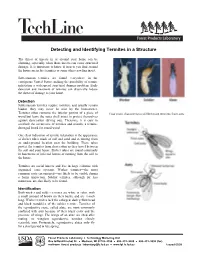
Detecting and Identifying Termites in a Structure The threat of insects in or around your home can be alarming, especially when those insects can cause structural damage. It is important to know if insects you find around the house are in fact termites or some other crawling insect. Subterranean termites are found everywhere in the contiguous United States, making the possibility of termite infestation a widespread structural damage problem. Early detection and treatment of termites can drastically reduce the threat of damage to your home. Detection Subterranean termites require moisture and usually remain hidden—they may never be seen by the homeowner. Termites often consume the interior portion of a piece of Four main characteristics differentiate termites from ants: wood but leave the outer shell intact to protect themselves against desiccation (drying out). Therefore, it is easy to overlook the occurrence of termites and mistake a termite- damaged board for sound wood. One clear indication of termite infestation is the appearance of shelter tubes made of soil and sand and stemming from an underground location near the building. These tubes protect the termites from desiccation as they travel between the soil and your house. Shelter tubes are found commonly in basements of infected homes or running from the soil to the house. Termites are social insects and live in large colonies with organized caste systems. Worker termites—the most common caste encountered—are likely to be visible during a home inspection. Soldier termites, although far less numerous, are also likely to be found. Identification Both worker and soldier termites are white in color, with a small amount of brown on their backs, and are ¼-inch long. -

Evaluation of the Chemical Defense Fluids of Macrotermes Carbonarius
www.nature.com/scientificreports OPEN Evaluation of the chemical defense fuids of Macrotermes carbonarius and Globitermes sulphureus as possible household repellents and insecticides S. Appalasamy1,2*, M. H. Alia Diyana2, N. Arumugam2 & J. G. Boon3 The use of chemical insecticides has had many adverse efects. This study reports a novel perspective on the application of insect-based compounds to repel and eradicate other insects in a controlled environment. In this work, defense fuid was shown to be a repellent and insecticide against termites and cockroaches and was analyzed using gas chromatography-mass spectrometry (GC– MS). Globitermes sulphureus extract at 20 mg/ml showed the highest repellency for seven days against Macrotermes gilvus and for thirty days against Periplaneta americana. In terms of toxicity, G. sulphureus extract had a low LC50 compared to M. carbonarius extract against M. gilvus. Gas chromatography–mass spectrometry analysis of the M. carbonarius extract indicated the presence of six insecticidal and two repellent compounds in the extract, whereas the G. sulphureus extract contained fve insecticidal and three repellent compounds. The most obvious fnding was that G. sulphureus defense fuid had higher potential as a natural repellent and termiticide than the M. carbonarius extract. Both defense fuids can play a role as alternatives in the search for new, sustainable, natural repellents and termiticides. Our results demonstrate the potential use of termite defense fuid for pest management, providing repellent and insecticidal activities comparable to those of other green repellent and termiticidal commercial products. A termite infestation could be silent, but termites are known as destructive urban pests that cause structural damage by infesting wooden and timber structures, leading to economic loss. -

03 Literature Review
- Literature Review: - Sr. No .1 - Name of the Author : P.G. Koehler and C. L. Tucker - Name of the Article : Subterranean Termite - Pages: 7 Nos. - Date of Published : April 1993 Revised in 2003 University of Florida, IFAS, Extention No. ENY 210 - Journal Name : Entomology and Nematology, Florida Cooperative Service - Review: Subterranean Termite, Dampwood and Drywood Termite are the main three types of termites . Subterranean Termites are the most destructive and it attacks structures by building tubes from nest to wood structures. They feed on wood which contain cellulose which is digested by them by protozoa and convert cellulose into usable food. The nest in the soil contains moisture to protect them from low humidity and predation and they build mud tunnels or tubes to reach wood. At swarming stage the broken wings are located at light are hence they are attracted to light. No wood contact with soil, ventilation, regular inspection can avoid the entry of termite and for effective control for Subterranean termite, Pre and post treatment methods for new and for existing structures at different stages with repellent and non repellent insecticides as required with the additional control method of using Termite Bait. - Sr. No . 2 - Name of the Author : Matthew R. Tarver, Christopher B. Florane, Dunhua Zhang, Casey Grimm and Alan R. Lax - Name of the Article : Methoprene and temperature effects on caste differentiation and protein composition in the Formosan Subterranean termite, Coptotermesfomosanus - Pages: 10 Nos. - Date of Published : December 2011 - Journal Name : Journal of Insect Science, Vol. 12/ article 18 - Review: Worker to soldier differentiation is modulated by temperature i.e. -

Intracolonial Demography of the Mound-Building Termite Macrotermes Natalensis (Haviland) (Isoptera, Termitidae) in the Northern Kruger National Park, South Africa
Insectes soc. 47 (2000) 390–397 0020-1812/00/040390-08 $ 1.50+0.20/0 Insectes Sociaux © Birkhäuser Verlag, Basel, 2000 Research article Intracolonial demography of the mound-building termite Macrotermes natalensis (Haviland) (Isoptera, Termitidae) in the northern Kruger National Park, South Africa V.W. Meyer 1, *, R.M. Crewe 1,L.E.O.Braack2, H.T. Groeneveld 3 and M.J. van der Linde 3 1 Department of Zoology and Entomology, University of Pretoria, Pretoria, 0002, South Africa, e-mail: [email protected]; [email protected] 2 Department of Conservation Development, Kruger National Park, Skukuza, 1350, South Africa, e-mail: [email protected] 3 Department of Statistics, University of Pretoria, Pretoria, 0002, South Africa, e-mail: [email protected]; [email protected] * Correspondence address: PO Box 1969, Wingate Park, 0153, South Africa Received 14 January 2000; revised 18 September 2000; accepted 26 September 2000. Summary. This paper reports on the number of individuals todeally (from the rectum). Secondly, termites have been in Macrotermes natalensis (Hav.) colonies of different sized shown to fix nitrogen (Curtis and Waller, 1998). If the nitro- mounds in the northern Kruger National Park. Mounds were gen fixation rate per individual termite is known, caste num- fully excavated, termites collected by means of vacuuming, bers and proportions provided by the present study can be and colony size estimated by sub-sampling. The proportion used to accurately derive overall nitrogen fixation, as rates of of termites in the mound (above and underground sections) fixation vary among species and castes via microbes and amounts to more than 70% of the colony; the rest being pre- fungi (e.g., Matsumoto and Abe, 1979; Collins, 1983). -

Complementary Symbiont Contributions to Plant Decomposition in a Fungus-Farming Termite
Complementary symbiont contributions to plant decomposition in a fungus-farming termite Michael Poulsena,1,2, Haofu Hub,1, Cai Lib,c, Zhensheng Chenb, Luohao Xub, Saria Otania, Sanne Nygaarda, Tania Nobred,3, Sylvia Klaubaufe, Philipp M. Schindlerf, Frank Hauserg, Hailin Panb, Zhikai Yangb, Anton S. M. Sonnenbergh, Z. Wilhelm de Beeri, Yong Zhangb, Michael J. Wingfieldi, Cornelis J. P. Grimmelikhuijzeng, Ronald P. de Vriese, Judith Korbf,4, Duur K. Aanend, Jun Wangb,j, Jacobus J. Boomsmaa, and Guojie Zhanga,b,2 aCentre for Social Evolution, Department of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark; bChina National Genebank, BGI-Shenzen, Shenzhen 518083, China; cCentre for GeoGenetics, Natural History Museum of Denmark, University of Copenhagen, DK-1350 Copenhagen, Denmark; dLaboratory of Genetics, Wageningen University, 6708 PB, Wageningen, The Netherlands; eFungal Biodiversity Centre, Centraalbureau voor Schimmelcultures, Royal Netherlands Academy of Arts and Sciences, NL-3584 CT, Utrecht, The Netherlands; fBehavioral Biology, Fachbereich Biology/Chemistry, University of Osnabrück, D-49076 Osnabrück, Germany; gCenter for Functional and Comparative Insect Genomics, Department of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark; hDepartment of Plant Breeding, Wageningen University and Research Centre, NL-6708 PB, Wageningen, The Netherlands; iDepartment of Microbiology, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria SA-0083, South Africa; and jDepartment of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark Edited by Ian T. Baldwin, Max Planck Institute for Chemical Ecology, Jena, Germany, and approved August 15, 2014 (received for review October 24, 2013) Termites normally rely on gut symbionts to decompose organic levels-of-selection conflicts that need to be regulated (12). -

Spatial Distribution and Density of Termite Mounds in a Protected Habitat in the South of Cote D’Ivoire: Case of National Floristic Center (Cnf) of Ufhb of Abidjan
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by European Scientific Journal (European Scientific Institute) European Scientific Journal January 2015 edition vol.11, No.3 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 SPATIAL DISTRIBUTION AND DENSITY OF TERMITE MOUNDS IN A PROTECTED HABITAT IN THE SOUTH OF COTE D’IVOIRE: CASE OF NATIONAL FLORISTIC CENTER (CNF) OF UFHB OF ABIDJAN Boga Jean-Pierre, PhD Assistant-Prof. Akpesse Akpa Alexandre Moise, PhD Assistant-Prof. Ouali-N’goran San-Whouli Mauricette, PhD Associate-Prof. Laboratory of Zoology and Animal Biology, UFR-Biosciences, Félix Houphouët-Boigny University of Abidjan, Côte d’Ivoire Trabi Crolaud Sylvain, PhD Assistant Laboratory of Animal Biology, UFR of agroforestery, Jean-Lourougnon Guédé University of Daloa, Côte d'Ivoire Kouassi Kouassi Philippe, PhD Prof. Tano Yao, PhD Prof. Yapi Ahoua, PhD Associate-Prof. Laboratory of Zoology and Animal Biology, UFR-Biosciences, Félix Houphouët-Boigny University of Abidjan, Côte d’Ivoire Abstract The spatial distribution and termite mounds density and their activity were studies in order to assess to the biological restoration level in a protected area, knowing that termites are considered as tropical ecosystem engineers. The CNF area was subdivided into 4 sectors (SW, SE, NW and NE). In every sector of 1.75 ha, 20 transects (5 m x 100 m) were sampled. Termite nests were counted. Their dimensions and geographical coordinates were recorded. The superposition of spatial distribution maps of the 3 types of termite mounds showed an impressive abundance of termite mounds in all CNF area. In total, there were recorded 165 termite mounds. -

Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected]
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2014 Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Wang, Cai, "Research in Biological Control of the Formosan Subterranean Termite" (2014). LSU Doctoral Dissertations. 598. https://digitalcommons.lsu.edu/gradschool_dissertations/598 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. RESEARCH IN BIOLOGICAL CONTROL OF THE FORMOSAN SUBTERRANEAN TERMITE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Cai Wang M.S., Chinese Academy of Science, 2010 B.S., Huazhong University of Science and Technology, 2007 August 2014 ACKNOWLEDGMENTS I would like to express my sincerest appreciation to my major professor, Dr. Gregg Henderson, a very important person in my life. I am very impressive for his meticulous attitude for scientific research. I benefited greatly from his valuable and illuminating suggestions for my research. It is also very touching for Dr. Henderson’s patience for my preliminary and sometimes “crazy” ideas. Also, he always could “see” what I ignored. For example, when I unintentionally talked about an observation that soldier and worker termites run in different directions after disturbance, he immediately pointed out the potential value to continue studying this and gave me valuable suggestions in the experiment. -

Totally Termites (Grades 3 – 5)
Totally Termites (Grades 3 – 5) Lesson Overview Students will explore the world of termites. This lesson includes a close-up look at termite specimens, special termite adaptations and insect anatomy. Students will also learn about property risks associated with termites, and how pest control professionals manage termite problems. Correlation with National Science Teachers Association (NSTA) § History and Nature of Science: Content Standard G: Science as a human endeavor § Life Science: Content Standard C: The characteristics of organisms § Science as Inquiry: Content Standard A: Abilities necessary to do scientific inquiry § Science in Personal and Social Perspectives: Content Standard F: Types of Resources, Changes in Environments, Science and Technology in Local Challenges Key Concepts: Vocabulary Words: § Termites in the home are animals that are out of Adaptation place; they are considered pests. § Termites are amazing insects; they have unique Insect mouthparts, a special adaptation, that helps them Mouthpart survive. § Termites have specific body features that make them Pest insects. Pest Management § Termite behavior is influenced by hunger. Risk Specimen Skills Learned: Subterranean Termite Discussion Cause/Effect Communication Social Classification Appreciation Recognition Observation www.pestworldforkids.org page 1 of 10 Totally Termites (Grades 3 – 5) Getting Ready: Estimated Time: § Preparation: 20 Minutes § Lesson: 45 – 60 Minutes Materials: § Find specimens of the Subterranean Termite: Winged Reproductive Swarmers and Workers (see Additional Resources section for termite sources) § Hand Lenses (one per student is ideal) § Termite Fact Sheet § Termite Anatomy Sheet Preparation: § Arrange for and gather termite specimens (see Resources). One specimen per student is ideal. § Gather hand lenses (one per student is ideal). § Invite a termite control specialist into the classroom as a guest speaker. -

Telling Time with Termite Mounds: Termite Mound Declination, Azimuth, and Structure in Tarangire National Park
Telling Time with Termite Mounds: Termite mound declination, azimuth, and structure in Tarangire National Park Erin Frankson St. Olaf College Macrotermes michaelseni termites are found in savannah habitats throughout sub-Saharan Africa. Macrotermes michaelseni differ from other species of termites in that they construct chimney-shaped mounds in order to house their colonies (Turner 2001). These mounds are com- plex structures which utilize the sun as a tool for thermoregulation. By building their mounds at an angle, Macrotermes michaelseni are able to control air flow and regulate temperature within the inner chambers of their mounds. This study examined the zenith (heading on the hori- zon to which the mound points) and azimuth (the angle of tilt from ver- tical) of Macrotermes michaelseni termite mounds in Northern Tanza- nia. A total of 62 termite mounds were studied in and around Tarangi- re National Park during the month of October. Overall, the average zenith of Macrotermes michaelseni mounds was found to be 29.4 °, how- ever, average angle decreased as termite mound height increased. Of the 62 mounds studied, 59 faced northwest, with an average azimuth of 61.7 ° west of north. These results suggest that one can indeed use a termite mound in order to tell time. We tested this idea by constructing a sundial which mimicked the average angle and direction of the Ma- crotermes michaelseni termite mounds. We recorded the shadow cast by the mound for every hour of daylight and ultimately produced a chart which can be used to determine time using a Macrotermes michaelseni termite mound. Overall, termites provide vital ecosystem services for the surrounding environment. -

Termite Communities Along a Disturbance Gradient in a West African Savanna
insects Article Termite Communities along A Disturbance Gradient in a West African Savanna Janine Schyra 1,* and Judith Korb 1,2 1 Behavioral Biology, University of Osnabrueck, Barbarastr. 11, D-49076 Osnabrueck, Germany; [email protected] 2 Evolution and Ecology, Albert-Ludwigs-University Freiburg, Hauptstr. 1, D-79104 Freiburg im Breisgau, Germany * Correspondence: [email protected] Received: 23 November 2018; Accepted: 30 December 2018; Published: 8 January 2019 Abstract: (1) Background: Termites are important ecosystem engineers, crucial for the maintenance of tropical biodiversity and ecosystem functioning. But they are also pests which cause billions of dollars in damage annually to humans. Currently, our understanding of the mechanisms influencing species occurrences is limited and we do not know what distinguishes pest from non-pest species. (2) Method: We analyzed how anthropogenic disturbance (agriculture) affects species occurrences. We tested the hypothesis that strong disturbance functions as a habitat filter and selects for a subset of species which are major pests of crop. Using a cross-sectional approach, we studied termite assemblage composition along a disturbance gradient from fields to 12-year-old fallows in a West African savanna. (3) Results: We reliably identified 19 species using genetic markers with a mean of about 10 species—many of them from the same feeding type—co-occurring locally. Supporting our hypothesis, disturbance was associated with environmental filtering of termites from the regional species pool, maybe via its effect on vegetation type. The most heavily disturbed sites were characterized by a subset of termite species which are well-known pests of crop. -

FUDMA Journal of Sciences (FJS) Vol. 4 No. 2, June, 2020, Pp 92 - 100 92 EFFECT of PHYSICO-CHEMICAL… Akpan, Et Al., FJS
FUDMA Journal of Sciences (FJS) EFFECT OF PHYSICO-CHEMICAL… ISSNAkpan, online: et al., 2616 -1370 FJS ISSN print: 2645 - 2944 Vol. 4 No. 2, June, 2020, pp 92 -100 DOI: https://doi.org/10.33003/fjs -2020-0402-206 EFFECT OF PHYSICO-CHEMICAL PARAMETERS ON THE ABUNDANCE AND DIVERSITY OF TERMITES AND OTHER ARTHROPODS IN TERMITE MOUNDS IN UYO, AKWA IBOM STATE, NIGERIA. *1Akpan, Akaninyene Udoh, 2Ojianwuna, Chioma Cynthia, 1Ubulom, Peace Mayen Edwin, 1Clement Ameh Yaro, 1Oboho, Diligent Efiong. 1Entomology Unit – Department of Animal and Environmental Biology, University of Uyo, Uyo, Akwa Ibom State, Nigeria. 2Department of Animal and Environmental Biology, Delta State University, Abraka. Delta State. Nigeria *Corresponding author e-mail: [email protected] ABSTRACT Termites are generally regarded as pests, although they have some beneficial roles to play in the ecosystem, particularly in the soil. This study was conducted between January 2018 and April 2018, to determine the effect of physico-chemical parametrs on abundance and diversity of termites and other arthropods in termite mounds in Uinversity of Uyo Community. Soil samples were randomly collected from six termite mounds from two sites for physiochemical parameters analysis and these were temperature, pH, moisture content, nitrogen, phosphorus, magnesium, copper, sodium, potassium, manganese and iron.. The termites and other arthropods were preserved in 70% ethanol. Temperature and moisture content, copper, sodium and iron were significant. The results revealed that the physicochemical parameters affected the termite species abundance as station 1 (539) had relatively more of the termite species than station 2 (551), and also affected the diversity of the termites as station 1 (0.89) had relatively more diversity of the termites than station 2 (0.66). -

Social Insects: Bees, Wasps, Ants and Termites
Enrico Bonatti E.d.T. Outreach Practicum 11/11/2011 Social Insects: Bees, Wasps, Ants and Termites Teacher Resource Guide Before one understands what ‘social’ insects are it is first important to understand what an insect is in the first place. Insects are small arthropods with six legs and usually one or two pairs of wings; they are placed in the class Insecta or Hexapoda. The have an exoskeleton divided in three parts; head, thorax and abdomen. What are Social Insects? The eusocial, or most social of the insects, are the bees, wasps, ants, and termites. The bees, wasps, and ants are in the Order Hymenoptera, while the termites are in the Order Isoptera. All of the ants and termites are eusocial, while both the bees and wasps have many species that live solitary lives. Certain traits characterize the eusocial insects: -Reproductive division of labor: this means that the queen reproduces almost exclusively while other members of the colony specialize on different tasks. -Cooperative brood care: this means that contrastingly to many different animals, social insects all tend the brood together indiscriminately of whose offspring it is. -In the ants and termites there are castes that carry out different functions necessary for the survival of the colony depending on the size or age of the insect they carry different functions Social insects are organized into different ‘castes’, these are characterized by specialized roles (queen, soldiers, workers and such). Usually there is a queen that produces a lot of offspring and apart from her, many other sterile daughters (workers) that depending on their age or structure carry out specific tasks in or around the colony.