Sean S. Davies, Ph.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
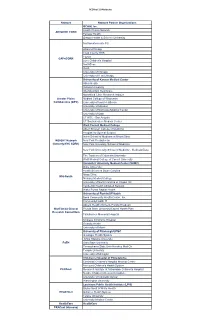
Sites in Pcornet
PCORnet 2.0 Networks Network Network Partner Organizations OCHIN, Inc. Health Choice Network ADVANCE CDRN Fenway Health Oregon Health & Science University Northwestern (site PI) AllianceChicago Cook County HHS Loyola CAPriCORN Lurie Children's Hospital NorthShore Rush University of Chicago University of IL at Chicago University of Kansas Medical Center Allina Health Indiana University InterMountain Healthcare Marshfield Clinic Research Institute Greater Plains Medical College of Wisconsin Collaborative (GPC) University of Iowa Healthcare University of Missouri University of Nebraska Medical Center University of Utah UT HSC - San Antonio UT Southwestern Medical Center Weill Cornell Medical College Albert Einstein College of Medicine Hospital for Special Surgery Icahn School of Medicine at Mount Sinai INSIGHT Network New York Presbyterian (formerly NYC CDRN) New York University School of Medicine New York University School of Medicine - Medicaid Data The Trustees of Columbia University Weill Medical College of Cornell University Vanderbilt University Medical Center (VUMC) Duke University Health Sciences South Carolina Mayo Clinic Mid-South Meharry Medical College University of North Carolina at Chapel Hill Vanderbilt Health Affiliated Network Wake Forest Baptist Health University of Florida/UFHealth Bond Community Health Center, Inc. CommunityHealth IT Advent Health (formerly Florida Hospital) OneFlorida Clinical Florida State University/Capital Health Plan Research Consortium Tallahassee Memorial Hospital Nicklaus Children's Hospital Orlando -

University of Utah
University of Utah Program Information Program Name University of Utah General Description / Special Programs Established 1969 Country United States State UT City Salt Lake City Address Line 1 Department of Physical Therapy & Athletic Training Address Line 2 520 Wakara Way Address Line 3 Zip 84108 Fax Phone1 801-585-9510 Phone2 801-581-8681 Email1 [email protected] Email2 Email3 Website https://health.utah.edu/physical-therapy-athletic-training/degree-programs/physical-therapy Program Information The University of Utah Doctor of Physical Therapy program is ranked among the top 10% of all PT programs in the United States. Our program in physical therapy has been preparing students to be highly competent and compassionate professionals for over 50 years. We take pride in bringing together the most advanced educational philosophies and the most current research together with the finest faculty to create an effective and supportive learning environment. We treat students as professionals, requiring personal accountability. We are fully accredited by the Commission on Accreditation in Physical Therapy Education. The program admits 50 students each year. Courses cover 9 semesters over 3 years culminating in an entry-level Doctor of Physical Therapy (DPT) Degree. Since 2008, our graduates have achieved a 99% first-time pass rate on the national license exam. Employment rates are 100% as reported by graduates actively seeking practice positions (4-year average). Full-time faculty includes ABPTS specialists, master clinicians in neurological rehabilitation, orthopedic and sports, infectious disease, burn and wound care, as well as education. Program Description The University of Utah Physical Therapy Department houses three research laboratories: The Skeletal Muscle Exercise Research Facility, the Clinical Neuromuscular Laboratory, and the Motion Capture Core Facility. -

Chapter 17 University of Utah Part 1 Educational Telecommunications
Utah Code Chapter 17 University of Utah Part 1 Educational Telecommunications 53B-17-101 Legislative findings on public broadcasting and telecommunications for education. The Legislature finds and determines the following: (1) The University of Utah's Dolores Dore' Eccles Broadcast Center is the statewide public broadcasting and telecommunications facility for education in Utah. (2) The center shall provide services to citizens of the state in cooperation with higher and public education, state and local government, and private industry. (3) Distribution services provided through the center shall include KUED - TV, KUER - FM, and KUEN - TV. (4) KUED - TV and KUER - FM are licensed to the University of Utah. (5) The Utah Education and Telehealth Network's broadcast entity, KUEN - TV, is licensed to the Utah Board of Higher Education and, together with UETN, is operated on behalf of the state's systems of public and higher education. (6) All the entities referred to in Subsection (3) are under the administrative supervision of the University of Utah, subject to the authority and governance of the Utah Board of Higher Education. (7) This section neither regulates nor restricts a privately owned company in the distribution or dissemination of educational programs. Amended by Chapter 365, 2020 General Session 53B-17-101.5 Definitions. As used in this part: (1) "Board" means the Utah Education and Telehealth Network Board. (2) "Education Advisory Council" means the Utah Education Network Advisory Council created in Section 53B-17-107. (3) "Digital resource" means a digital or online library resource, including a database. (4) "Digital resource provider" means an entity that offers a digital resource to customers for license or sale. -
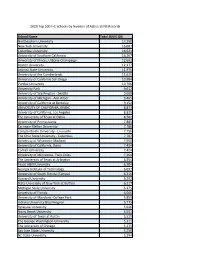
School Name Total SEVIS IDS Northeastern University
2020 Top 500 F-1 Schools by Number of Active SEVIS Records School Name Total SEVIS IDS Northeastern University 17,290 New York University 16,667 Columbia University 16,631 University of Southern California 16,207 University of Illinois, Urbana-Champaign 12,692 Boston University 12,177 Arizona State University 11,975 University of the Cumberlands 11,625 University of California San Diego 10,984 Purdue University 10,706 University Park 9,612 University of Washington - Seattle 9,608 University of Michigan - Ann Arbor 9,465 University of California at Berkeley 9,152 UNIVERSITY OF CALIFORNIA, IRVINE 8,873 University of California, Los Angeles 8,825 The University of Texas at Dallas 8,582 University of Pennsylvania 7,885 Carnegie Mellon University 7,786 Campbellsville University - Louisville 7,756 The Ohio State University - Columbus 7,707 University of Wisconsin-Madison 7,550 University of California, Davis 7,434 Cornell University 7,424 University of Minnesota, Twin Cities 7,264 The University of Texas at Arlington 6,954 Texas A&M University 6,704 Georgia Institute of Technology 6,697 University of South Florida (Tampa) 6,316 Harvard University 6,292 State University of New York at Buffalo 6,217 Michigan State University 6,175 University of Florida 6,065 University of Maryland -College Park 5,859 Indiana University Bloomington 5,775 Syracuse University 5,646 Stony Brook University 5,591 University of Texas at Austin 5,529 The George Washington University 5,311 The University of Chicago 5,275 San Jose State University 5,250 NC State University 5,194 Harrisburg University of Science & Tech 5,127 University of Illinois at Chicago 5,120 Stanford University 4,983 Duke University & Health Sys. -

Utah Vs. "Arizona State MECOMING
OFFICIAL PROGRAM 50 * Utah vs. "Arizona State MECOMING IN THIS ISSUE: Who That Horse Is" Tomorrow, Sunday, Nov. 5 Chicago vs. Detroit 11:00 a. m. New York vs. Minnesota 1:30 p. m. • • ' MOUNTAIN AMERICA'S NUMBER ONE SPORTS STATION the big f>Jay : The big play these days is to Hotel Utah. And little wonder! It's all new, from the ground up. New chandeliers, new furniture, new carpets, new draperies, new lighting and fresh new colors everywhere. Food? The best! Dancing? You bet! Ted Johnson and his orchestra are back for the Fall and Winter season. Sunday Brunch, too — and the musical fashion show luncheon each Monday. Make the big play. Live it up! Why not start tonight? Hotel Utah New again... and fresh as a flower! H. N. (Hank) Aloia, Managing Director OFFICIAL PROGRAM OFFICIAL WATCH TABLE OF CONTENTS FOR THIS GAME Today's Game __ 2 "Welcome Alumni" President James C. Fletcher 3 •**••** Dr. G. Homer Durham, President, Arizona State University 4 Clyde B. Smith, Athletic Director, Arizona State University 4 LONGINES The Arizona State Campus 7 THE WORLD'S Utah Alumni Association, (Utah Man) 8 MOST HONORED Utah Marching Band 9 WATCH® Head Coach Frank Kush, Arizona State 10 10 world's fair grand prizes Meet the Sun Devils ...11, 13, 15, 17 28 gold medals w Arizona State Assistants 12 Arizona State Alphabetical Roster 21 Longines watches are recognized as OFFICIAL for timing world "Who That Horse Is" Roy McHugh 22 championships and Olympic sports Arizona State Seven Game Statistics 23 in all fields throughout the world. -

FINAL Utah Legislature 2020
President Ruth V. Watkins Higher Ed Appropriations Subcommittee | Feb. 10, 2020 Our Progress 2023 Historic graduation rates, major faculty awards, and sponsored project awards 2018 80% Graduation Rate 1,200 Major Faculty Awards 2013 70% Graduation Rate $650M 928 Sponsored Project Awards 60% Major Faculty Awards Graduation Rate $515M 654 Sponsored Project Awards Major Faculty Awards $388M Sponsored Project Awards The University of Utah is NOW recognized as one of America’s leading research universities AAU Publics: Tuition & Fees (2019) Best Public Universities 2020 1. University of California, Los Angeles 4. University of California, Berkeley 7. University of Washington 33. University of Utah 35. University of Arizona 40. University of Colorado Boulder 43. Washington State University (t) 59. Arizona State University 62. University of Oregon (t) 82. Oregon State University (t) Wall Street Journal/Times Higher Education 1200 Engineering Degrees Granted 1,120 SB 61 : Increase the number of engineering and computer 1000 science graduates to advance the “well-being of the State and its citizens.” 800 SB 61 Passed 600 366 400 200 0 0 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 STEM Degrees Awarded 2018 The University of 2,245 2,310 4,555 Utah produces half 2017 of all STEM 2,106 2,217 4,323 degrees awarded in the State of Utah 2016 1,880 1,992 3,872 by public institutions 2015 1,630 1,829 3,459 2014 1,678 1,768 3,446 2013 1,280 1,592 2,872 2012 1,425 1,553 2,978 University of Utah Other USHE institutions combined William Nursery sales NEW! Master of Software Development Pluralsight Data streaming infrastructure engineer Sydney Ted Junchen Chemistry teacher National Security Agency Broadcom Inc. -

Curriculum Vitae
law.yale.edu http://www.law.yale.edu/faculty/rosecurriculumvitae.htm Curriculum Vitae CAROL MARGUERITE ROSE Yale Law School Box 208215 New Haven, Connecticut 06520-8215 (203) 432-7309. E-mail:[email protected] and University of Arizona College of Law Box 210176 Tucson Arizona 85721-0176 (520) 621-5544 Email [email protected] EDUCATION 1973-75, 1976-77, UNIVERSITY OF CHICAGO LAW SCHOOL, Chicago, Illinois. J.D. June 1977. Graduated with honors. 1965-69, CORNELL UNIVERSITY, Ithaca, New York. Ph.D., History, 1970. Major concentration European social & constitutional history; minor American Constitutional Law. 1962-63, UNIVERSITY OF CHICAGO, Chicago, Illinois. M.A. Political Science, 1963. 1958-62, ANTIOCH COLLEGE, Yellow Springs, Ohio. B.A., Philosophy, 1962. Third year in Germany, studied at University of Tübingen.) WORK EXPERIENCE 2005 - ASHBY LOHSE PROFESSOR OF WATER AND NATURAL RESOURCE LAW, UNIVERSITY OF ARIZONA COLLEGE OF LAW, Tucson, Arizona 2005- GORDON BRADFORD TWEEDY PROFESSOR OF LAW AND ORGANIZATION, EMERITA, AND PROFESSORIAL LECTURER, YALE LAW SCHOOL, New Haven, Connecticut 1989-2005, GORDON BRADFORD TWEEDY PROFESSOR OF LAW AND ORGANIZATION, YALE LAW SCHOOL, New Haven, Connecticut. (formerly Fred A. Johnston Professor) Spring 2002, VISITING PROFESSOR OF LAW, New York University School of Law, New York, New York Spring 1997, VISITING PROFESSOR OF LAW, University of Arizona School of Law, Tucson, Arizona Spring 1992, FUJIYAMA DISTINGUISHED VISITING PROFESSOR OF LAW University of Hawaii, Honolulu Summer 1990, DISTINGUISHED VISITING SCHOLAR, UNIVERSITY OF ADELAIDE SCHOOL OF LAW, Adelaide, Australia l982-1989, LEWIS AND HARRIET ANCEL PROFESSOR OF LAW AND PUBLIC POLICY, NORTHWESTERN UNIVERSITY SCHOOL OF LAW, Chicago, Illinois. -

Furlough and Finance at Uarizona in the Wake of COVID-19
General Faculty Financial Advisory Committee Report (1) Furlough and Finance at UArizona in the Wake of COVID-19 Presented to the University of Arizona Executive Leadership Team Presented by the General Faculty Financial Advisory Committee (GFFAC) August 6, 2020 General Faculty Financial Advisory Committee Report (2) Table of Contents Acknowledgements .............................................................................................................................4 Committee Description and Charge...................................................................................................5 Creation of GFFAC.............................................................................................................................................. 5 Committee Duration ........................................................................................................................................... 5 Committee Members.......................................................................................................................................... 6 Committee Charge ............................................................................................................................................. 6 Report Goals ......................................................................................................................................................... 8 Research Options and Provide Recommendations .......................................................................... 8 Increase Understanding -

Medical School Graduates Program 2020
Recognition of the Class of 2020 Graduates Program PROCESSIONAL (Guests remain seated) Trumpet Tune John Stanley OPENING REMARKS RECOGNITION OF FACULTY AND STUDENTS Jeffrey R. Balser, M.D., Ph.D. President and CEO, Vanderbilt University Medical Center Dean, Vanderbilt University School of Medicine MESSAGE TO THE GRADUATES Donald W. Brady, M.D. Senior Associate Dean for Health Sciences Education Executive Vice President for Educational Affairs, Vanderbilt University Medical Center CHARGE AND CONFERRAL OF DEGREES CONFERRAL OF ACADEMIC HOOD Jeffrey R. Balser, M.D., Ph.D. PRESENTATION OF GRADUATES A. Scott Pearson, M.D. Professor of Surgery RECOGNITION OF THE DOCTOR OF MEDICINE OATH Jeffrey R. Balser, M.D., Ph.D. RECESSIONAL (Guests please stand) Hornpipe from Water Music George Frideric Handel Contents Faculty Recognition ....................................................................2 School Administration Ceremony Honorees Emeritus Professors Program Directors College Mentors Portfolio Coaches Academic Regalia .......................................................................5 Degrees Conferred ......................................................................6 Doctor of Medicine .....................................................................6 May 2020 Doctor of Medical Physics ..............................................................10 August 2019 Doctor of Audiology ...................................................................10 May 2020 Master of Education of the Deaf .........................................................10 -

Bibliography of Geothermal Characteristics of the Roosevelt Hot Springs System and Adjacent Forge Egs Site, Milford, Utah
BIBLIOGRAPHY OF GEOTHERMAL CHARACTERISTICS OF THE ROOSEVELT HOT SPRINGS SYSTEM AND ADJACENT FORGE EGS SITE, MILFORD, UTAH Miscellaneous Publication 169-O Utah Geological Survey a division of UTAH DEPARTMENT OF NATURAL RESOURCES This bibliography is part of Geothermal Characteristics of the Roosevelt Hot Springs System and Adjacent FORGE EGS Site, Milford, Utah. https://doi.org/10.34191/MP-169 Bibliography of geothermal characteristics of the Roosevelt Hot Springs system and adjacent FORGE EGS site, Milford, Utah O1 BIBLIOGRAPHY OF GEOTHERMAL CHARACTERISTICS OF THE ROOSEVELT HOT SPRINGS SYSTEM AND ADJACENT FORGE EGS SITE, MILFORD, UTAH This bibliography of papers on the RHS and the FORGE site has been compiled because of the “gray” literature, such as conference proceedings, of most of the work. The format of these references is not uniform because of the differing databases that they have been pulled from. Active links for almost all publications have been inserted. In the case of copyrighted mate- rial the link references the appropriate journal. Allis, R.G., and Larsen, G., 2012, Roosevelt Hot Springs Geothermal field, Utah—reservoir response after more than 25 years of power production. Proc. 37th Workshop on Geothermal Reservoir Engineering, Stanford University, 8 p., https://pan- gea.stanford.edu/ERE/pdf/IGAstandard/SGW/2012/Allis.pdf. Allis, R.G., Gwynn, M., Hardwick, C., Kirby, S., Moore, J., and Chapman, D., 2015, Re-evaluation of the pre-development thermal regime of Roosevelt Hot Springs geothermal system, Utah. Stanford Geothermal Reservoir Engineering Work- shop. https://www.geothermal-library.org/index.php?mode=pubs&action=view&record=8019519. Allis, R.G., 2014, Formation pressure as a potential indicator of high stratigraphic permeability. -

University/College Degree Programs Contact Person(S)
University/College Degree Programs Contact person(s) Credentials Title(s) Contact Phone(s) Contact Email(s) Address City State Zip Additional Info Arizona State University DNP Program Patricia Janicek PhD, RN, WHNP-C Clinical Professor (602) 496-0893 [email protected] ASU Edson College of Nursing & Health Phoenix AZ 85004 https://nursingandhealth.asu.edu/degree- Innovation programs/graduate/womens-health-nurse- practitioner-dnp 500 North 3rd St Mail Code 3020 Debra Ilchak CNE, FAAN, FAANP Clinical Associate Professor (602) 496-0893 [email protected] ASU Edson College of Nursing & Health Phoenix AZ 85004 https://nursingandhealth.asu.edu/degree- Innovation programs/graduate/womens-health-nurse- practitioner-dnp 500 North 3rd St Mail Code 3020 Boston College Master's, Post- Allyssa Harris PhD, RN, WHNP-BC Assistant Professor and (617) 552-0550 [email protected] William F. Connell School Of Nursing, Chestnut Hill MA 02467 http://www.bc.edu/bc- Master's, PhD WHNP Program Director Boston College web/schools/cson/academics/masters- 140 Commonwealth Ave program/advanced-practice-nursing- Cushing Hall 302 specialties/womens-health-nurse- practitioner.html California State MSN, Post-Master's, Ruth Mielke PhD, CNM, (657) 278-2670 [email protected] California State University- Fullerton School Fullerton CA 92834-6868 http://nursing.fullerton.edu/programs/msn University-Fullerton DNP, MSN Women’s FACNM,WHNP of Nursing, EC-190 whc/ (Dual) Health Care 800 N. State College Blvd. P.O.Box 6868 California State MSN, Post-Master's, Pamela -

Financial Statements June 30, 2018 (With Independent
KUEN (A Public Telecommunications Department of the University of Utah) Financial Statements June 30, 2018 (With Independent Auditors’ Report Thereon) KUEN (A Public Telecommunications Department of the University of Utah) Table of Contents Page(s) Independent Auditors’ Report 3 – 5 Management’s Discussion and Analysis 6 – 10 Statement of Net Position 11 Statement of Revenues, Expenses, and Changes in Net Position 12 Statement of Cash Flows 13 – 14 Notes to Financial Statements 15 – 26 Required Supplementary Information: Schedule of the Proportionate Share of the Net Pension Liability 28 Schedule of Employer Contributions 29 Supplementary Schedules: Combining Schedule of Programs – Statement of Net Position June 30, 2018 31 Combining Schedule of Programs – Statement of Net Position June 30, 2017 32 Combining Schedule of Programs – Statement of Revenues, Expenses, and Changes in Net Position Year ended June 30, 2018 33 Combining Schedule of Programs – Statement of Revenues, Expenses, and Changes in Net Position Year ended June 30, 2017 34 Independent Auditors’ Report on Internal Control Over Financial Reporting and on Compliance and Other Matters Based on an Audit of Financial Statements Performed in Accordance with Government Auditing Standards 35 – 36 INDEPENDENT AUDITORS’ REPORT KUEN The University of Utah Board of Trustees and Ruth V. Watkins, President Salt Lake City, Utah We have audited the accompanying financial statements of KUEN (a public telecommunications department of University of Utah) and affiliates, which comprise the statement of net position as of June 30, 2018, and the related statements of revenues, expenses, and changes in net position and cash flows for the year then ended, and the related notes to the financial statements.