Related Uranium Mineralization in the Athabasca Basin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Athabasca Basin Communities Renew Partnership with the Uranium Mining Industry
Athabasca Basin Communities Renew Partnership with the Uranium Mining Industry Saskatoon, Saskatchewan, Canada, June 21, 2016 . The Athabasca communities, Cameco Corporation (Cameco) and AREVA Resources Canada Inc. (AREVA) are proud to announce the signing of a collaboration agreement that builds upon an enduring partnership in the development of uranium resources in the Athabasca Basin. The Ya’Thi Néné (“Lands of the North” in Dene) collaboration agreement confirms the continued support of the communities historically and traditionally associated with the Cigar Lake, McClean Lake and Rabbit Lake uranium mining operations. The comprehensive and unique agreement builds on the existing relationships and commercial arrangements between Cameco, AREVA and the three First Nation communities of Black Lake, Fond du Lac and Hatchet Lake, and the four communities of Stony Rapids, Wollaston Lake, Uranium City and Camsell Portage. “The renewed partnership agreement gives the Athabasca communities certainty, to help ensure that the companies operate sustainably, bringing positive changes for the future generation.” Diane McDonald, lead negotiator for the Athabasca communities “By working with industry, people living in the north have found ways to enhance the capacity and vitality of their communities while protecting their traditional values and lands. This agreement assures that strong partnership will continue.” Tim Gitzel, president and CEO of Cameco “This agreement further solidifies our longstanding collaboration with these communities. It speaks to our joint vision and commitment to the prosperity of northern Saskatchewan for decades to come.” Vincent Martin, president and CEO of AREVA Resources Canada The Ya’Thi Néné collaboration agreement builds on the existing impact management agreement signed in 1999. -

Project Management for Decommissioning of Hope Brook
Uranium Development & Exploration The Athabasca Basin, Northern Saskatchewan July 2020 | Corporate Update Cautionary Statements & References This presentation and the information contained herein is designed to help you understand management’s current views, and may not be appropriate for other purposes. This presentation contains information relating to other companies and provincial infrastructure, and the plans and availability thereof, derived from third-party publications and reports which Denison believes are reliable but have not been independently verified by the Company. Certain information contained in this presentation constitutes “forward-looking information”, within the meaning of the United States Private Securities Litigation Reform Act of 1995 and similar Canadian legislation concerning the business, operations and financial performance and condition of Denison. Generally, these forward-looking statements can be identified by the use of forward-looking terminology such as “plans”, “expects”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, or “believes”, or the negatives and / or variations of such words and phrases, or state that certain actions, events or results “may”, “could”, “would”, “might” or “will be taken”, “occur”, “be achieved” or “has the potential to”. In particular, this presentation contains forward-looking information pertaining to the results of, and estimates, assumptions and projections provided in, the PFS, including future development methods and plans, market prices, costs -

Press Release Denison Announces Appointment Of
Denison Mines Corp. 1100 – 40 University Ave Toronto, ON M5J 1T1 www.denisonmines.com PRESS RELEASE DENISON ANNOUNCES APPOINTMENT OF RON HOCHSTEIN AS BOARD CHAIR AND WELCOMES NEW DIRECTORS Toronto, ON – May 11, 2021. Denison Mines Corp. (“Denison” or the “Company”) (DML: TSX, DNN: NYSE American) is pleased to announce the appointment of Mr. Ron F. Hochstein as Chair of the Board of Directors (“Board Chair”). Mr. Hochstein was elected Board Chair, and Mr. Brian Edgar was elected Lead Director, at a meeting of the Company’s independent directors following the Company’s Annual General Meeting. The Company is also pleased to welcome David Neuburger and Jennifer Traub to the Board of Directors. Ron Hochstein, Denison’s Board Chair, commented, “I am honoured to have been elected Chair of Denison’s Board by the Company’s independent directors. This is a very exciting and important time in the modern history of Denison – as the Company focuses on advancing its low-cost Phoenix In-Situ Recovery (‘ISR’) uranium development project, at a time when the uranium market is showing signs of a sustained recovery. Navigating years of challenging uranium markets, with a view of building a sustainable low-cost uranium mining company, has taken great vision from the Company’s Board and management. Accordingly, on behalf of the Board of Directors, I would like to thank Catherine Stefan and Jack Lundin for their contributions to the Board, and specifically express our appreciation to Ms. Stefan, the Company’s outgoing Board Chair, for her legacy of leadership and oversight of the Company during her six year term as Chair and Lead Director and 17 year tenure as a Director of the Company and its predecessor Denison Mines Inc.” David Cates, Denison Director and President & CEO, added, “We are also pleased to welcome the addition of two highly qualified new Directors to join Denison’s Board. -

Open House 2014 Abstract Volume Saskatchewan Geological Survey
December 1st to 3rd, 2014 Open House 2014 Abstract Volume Saskatchewan Geological Survey December 1 to 3, 2014 Open House 2014 Abstract Volume Saskatchewan Geological Survey Printed under the authority of the Government of Saskatchewan Although the Saskatchewan Ministry of the Economy has exercised all reasonable care in the compilation, interpretation, and production of this report, it is not possible to ensure total accuracy, and all persons who rely on the information contained herein do so at their own risk. The Ministry of the Economy and the Government of Saskatchewan do not accept liability for any errors, omissions or inaccuracies that may be included in, or derived from, this report. Cover: “Lying down on the job”. Saskatchewan Geological Survey junior geological assistant Levi Paradis, examining polygonal cracks of the Athabasca Group on the southwest shore of Johnson Island, Lake Athabasca. Cross-section of the cracks suggests an earlier desiccation event followed by a fluid escape event. Smaller desiccation cracks and sandstone ripples are present within the larger polygonal features. (UTM 613943 m E, 6577875 m N, NAD 83, Zone 12). This volume may be downloaded from: www.economy.gov.sk.ca/previousoh Saskatchewan Geological Survey ii Open House 2014, Abstract Volume Contents page Technical Session 1: Uranium Geoscience A Geophysical Expressions of Ore Systems, Not Deposits – Our Current Understanding ....................................................................................................................... Ken Witherly 2 A Basin Development, Lithogeochemistry and Mineralization of the Athabasca Basin, Canada................................................................................................ Paul Ramaekers 3 * Spectacular Conglomerates of the Northwest Athabasca Basin: An Overview ............... Sean A. Bosman 4 A Fault Architecture, Associated Structures and Uranium Mineralization, Eastern Athabasca Basin: A Provisional Empirical Classification .......................................................... -
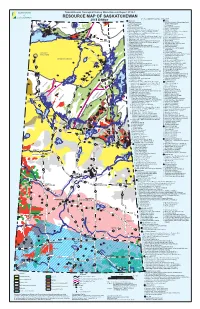
Mineral Resource Map of Saskatchewan
Saskatchewan Geological Survey Miscellaneous Report 2018-1 RESOURCE MAP OF SASKATCHEWAN KEY TO NUMBERED MINERAL DEPOSITS† 2018 Edition # URANIUM # GOLD NOLAN # # 1. Laird Island prospect 1. Box mine (closed), Athona deposit and Tazin Lake 1 Scott 4 2. Nesbitt Lake prospect Frontier Adit prospect # 2 Lake 3. 2. ELA prospect TALTSON 1 # Arty Lake deposit 2# 4. Pitch-ore mine (closed) 3. Pine Channel prospects # #3 3 TRAIN ZEMLAK 1 7 6 # DODGE ENNADAI 5. Beta Gamma mine (closed) 4. Nirdac Creek prospect 5# # #2 4# # # 8 4# 6. Eldorado HAB mine (closed) and Baska prospect 5. Ithingo Lake deposit # # # 9 BEAVERLODGE 7. 6. Twin Zone and Wedge Lake deposits URANIUM 11 # # # 6 Eldorado Eagle mine (closed) and ABC deposit CITY 13 #19# 8. National Explorations and Eldorado Dubyna mines 7. Golden Heart deposit # 15# 12 ### # 5 22 18 16 # TANTATO # (closed) and Strike deposit 8. EP and Komis mines (closed) 14 1 20 #23 # 10 1 4# 24 # 9. Eldorado Verna, Ace-Fay, Nesbitt Labine (Eagle-Ace) 9. Corner Lake deposit 2 # 5 26 # 10. Tower East and Memorial deposits 17 # ###3 # 25 and Beaverlodge mines and Bolger open pit (closed) Lake Athabasca 21 3 2 10. Martin Lake mine (closed) 11. Birch Crossing deposits Fond du Lac # Black STONY Lake 11. Rix-Athabasca, Smitty, Leonard, Cinch and Cayzor 12. Jojay deposit RAPIDS MUDJATIK Athabasca mines (closed); St. Michael prospect 13. Star Lake mine (closed) # 27 53 12. Lorado mine (closed) 14. Jolu and Decade mines (closed) 13. Black Bay/Murmac Bay mine (closed) 15. Jasper mine (closed) Fond du Lac River 14. -

Geology and Mineral Resources of the Athabasca Basin and Environs, Saskatchewan and Alberta W.L
Geology and Mineral Resources of the Athabasca Basin and Environs, Saskatchewan and Alberta W.L. Slimmon Saskatchewan Ministry of Energy and Resources [email protected] and D.I. Pană Alberta Energy Resources Conservation Board/Alberta Geological Survey, 4th Floor, Twin Atria Building, 49999-98th Ave, Edmonton, Alberta, Canada T6B 2X3 [email protected] Summary A new 1:500 000 scale compilation map of the Athabasca Basin and surrounding area has been recently published and will be displayed in the poster session at GeoCanada 2010. The base to the map is bedrock geology superimposed on a three-dimensional shaded relief topographic background. Another element is the locations of mineral occurrences which amongst others profile the region’s prolific uranium mineralization. Drillhole locations highlight where past exploration work has been done in the Athabasca Basin. Insets on the map detail the geology and mineral occurrences of selected areas and also include regional maps of aeromagnetic data and basement geology interpretations for the basin. Introduction The Athabasca Basin is host to the world’s largest high-grade unconformity-related uranium deposits. A recently published compilation map (Slimmon and Pană, 2010) represents the first large (1:500 000 scale), stand-alone map separate of the Athabasca Basin region in Saskatchewan and Alberta released since 1990 (Ramaekers, 1990). In addition to updated Athabasca Group geology, a 25 km wide band of the geology of the surrounding Canadian Shield provides some insight into the basement rocks. Also included are the locations of the region’s mineral resources and drillhole locations. The map shows the entire region at a scale that displays sufficient detail but is also a manageable size when printed. -

Mineralogical Characterization of Uranium Ores, Blends and Resulting Leach Residues from Key Lake Pilot Plant, Saskatchewan, Canada
MINERALOGICAL CHARACTERIZATION OF URANIUM ORES, BLENDS AND RESULTING LEACH RESIDUES FROM KEY LAKE PILOT PLANT, SASKATCHEWAN, CANADA A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Department of Geological Sciences University of Saskatchewan, Saskatoon, SK, Canada By MD. ALAUDDIN HOSSAIN Copyright Md. Alauddin Hossain, October 2014. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfilment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Geological Sciences University of Saskatchewan 114 Science Place Saskatoon, Saskatchewan Canada S7N 5E2 i ABSTRACT The production and storage of uranium mine mill tailings have the potential to contaminate local groundwater and surface waters with metals and metalloids. -

Uraninite-Bearing Granitic Pegmatite, Moore Lakes, Saskatchewan: Petrology and U-Th-Pb Chemical Ages
Uraninite-bearing Granitic Pegmatite, Moore Lakes, Saskatchewan: Petrology and U-Th-Pb Chemical Ages 1 1 1 3 Irvine R. Annesley , Catherine Madore , Richard T Kusmirski , and Tom Bonli Anm:slcv. l.R .. Madore. C .. Kusmirski, R.T.. and Bonli. T. (2000): lJraninite-bearing granitic pegmat1tc, Moore Lakes, . Saskatcl1ewan: Petrology and l!-Th-Pb chcmic,tl ages: in Summary of Investigations 2000, Volume 2. Saskatchewan Gcolog1cal Survey. Sask. Energy Mines, Misc. Rep. 2000-4.2. attribute their genesis to some variation of the Abstract diagenctic-hydrothermal model proposed by Hoeve This paper documents the.fir.1·t results ofa detailed and Sibbald ( 1978), which invokes the mixing of study of uraninite-bearing granitic pegmatite, which highly saline oxidized basinal fluids with variably occurs near unconj(Jrmity-lJpe uranium mineralizalion reduced basement fluids. More recent research shows in the Moore Lakes area in the southeastern part ofthe that the transport and precipitation of uranium is Athabasca Basin. Drilling has revealed that wanitic controlled by basin paleohydrology, basement pegmatites comprise <5 to 10% ofthe basement topography, large-scale reactivated basement cornplex with hallestimated being radioactive. structures, fluid flow, heat flow, and physiochemical Relatively fresh, radioactive granitic pegmatite, the traps (Hoeve and Quirt, 1984, 1987; Wilson and Kyser, subject of this investigation, was intersected in drill 1987; Kotzcr and Kyser, l 991, 1992. 1995; Fayek and hole ML00-08. The pegmatite is JO m thick and occurs Kyser, 1997; Quirt. 1997). There are several 55 m he/ow the unconformity with sandstones olthe hypotheses as to the source of the uranium. Pagel et al. -

Geology of the Uranium Deposits Related to the Sub-Athabasca Unconformity, Saskatchewan
GEOLOGICAL SURVEY OF CANADA COMMISSION GEOLOGIQUE DU CANADA PAPER 81-20 GEOLOGY OF THE URANIUM DEPOSITS RELATED TO THE SUB-ATHABASCA UNCONFORMITY, SASKATCHEWAN L.P. TREMBLAY Canada 1982 PAPER 81-20 GEOLOGY OF THE URANIUM DEPOSITS RELATED TO THE SUB-ATHABASCA UNCONFORMITY, SASKATCHEWAN L.P. TREMBLAY 1982 ©Minister of Supply and Services Canada 1982 Available in Canaoa through authorized bookstore agents and other bookstores or by mail from Canadian Government Publishing Centre Supply and Services Canada Hull, Quebec, Canada K1A 0S9 and from Geological Survey of Canada 601 Booth Street Ottawa, Canada K1A 0E8 A deposit copy of this publication is also available for reference in public libraries across Canada Cat. No. M44-81/20E Canada: $4.00 ISBN 0-660-11187-X Other countries: $4.80 Price subject to change without notice Original manuscript submitted: 1-2-80 Approved for publication: 27 - ft - 82 CONTENTS 1 Abstract/Resume 3 Introduction 3 Acknowledgments 3 General Geology 9 Description of deposits 9 Collins Bay - Michael Lake area 12 Rabbit Lake Mine 16 Raven and Horseshoe deposits 17 West Bear Deposit IS Collins Bay subarea IS Collins Bay A zone 21 Collins Bay B zone 21 Midwest Lake - McClean Lake area 22 Midwest Lake deposit 25 Dawn Lake deposits 26 McCJean Lake deposits 29 Key Lake area 29 Key Lake deposits 33 Carswell Circular Structure 3* Cluf f Lake deposits 38 Maurice Bay area 38 Maurice Bay deposit Northern rim of Athabasca Basin Stewart Island deposit 42 Fond-du-Lac deposit 46 Characteristics of the deposits 46 Common features 47 Differing features 49 Subtypes of Athabasca deposits 49 Conditions required to produce a deposit 49 Genesis of the deposits 51 Exploration 52 Bibliography Tables 5 1. -

Basement Lithologic Framework and Structural Features of the Western Athabasca Basin
Basement Lithologic Framework and Structural Features of the Western Athabasca Basin C.D. Card, J.E. Campbell, and W.L. Slimmon Card, C.D., Campbell, J.E., and Slimmon, W.L. (2003): Basement lithologic framework and structural features of the western Athabasca Basin; in Summary of Investigations 2003, Volume 2, Saskatchewan Geological Survey, Sask. Industry Resources, Misc. Rep. 2003-4.2, CD-ROM, Paper D-3, 17p. Abstract The final phase of field work of the Western Athabasca Basement Project included core logging in the Hook Lake and Carswell areas. The Careen Lake Group supracrustal package at Hook Lake is dominated by psammites and quartzites with subordinate psammopelites and migmatitic to diatexitic pelites. It is not known whether rare mafic rocks are part of, or intrude, the Careen Lake Group. All these are cut by peraluminous granites. In contrast, the Careen Lake Group in the Carswell Structure is dominated by psammopelites and migmatitic to diatexitic pelites, with subordinate psammites and quartzites. It is intruded by granodiorites to quartz diorites believed to be related to the ca. 1.986 to 1.960 Ga calc-alkaline intrusions of the Taltson Magmatic Zone and younger granites. The Careen Lake Group has been metamorphosed under upper amphibolite to granulite facies conditions and folded at least twice. On the other hand, the later granodiorites and quartz diorites appear to be more weakly deformed and less metamorphosed than the Careen Lake Group. This may be due to differences in the rheological properties of the rocks. It is certain that all of these rocks were metamorphosed at ca. -

Uranium Geoscience in the Athabasca Basin and Western Canada Sedimentary Basin in Northwestern Saskatchewan (NTS 74F and 74K)
Uranium Geoscience in the Athabasca Basin and Western Canada Sedimentary Basin in Northwestern Saskatchewan (NTS 74F and 74K) Sean A. Bosman 1, Jared Noll 2, Derek Johnson 3 and Brody Strocen 3 Information from this publication may be used if credit is given. It is recommended that reference to this publication be made in the following form: Bosman, S.A., Noll, J., Johnson, D. and Strocen, B. (2017): Uranium geoscience in the Athabasca Basin and Western Canada Sedimentary Basin in northwestern Saskatchewan (NTS 74F and 74K); in Summary of Investigations 2017, Volume 2, Saskatchewan Geological Survey, Saskatchewan Ministry of the Economy, Miscellaneous Report 2017-4.2, Paper A-4, 14p. Abstract The Patterson Lake region of northwest Saskatchewan, at the southwest margin of the Athabasca Basin, contains at least three high-grade uranium deposits, the Triple R, Arrow and Spitfire. This area is unique to the Athabasca region within Saskatchewan because of the presence of rocks of the Western Canada Sedimentary Basin, which overlie rocks of both the Taltson/Clearwater domains and the Athabasca supergroup. Phanerozoic stratigraphy in the area includes carbonate-rich rocks of the Lower to Middle Devonian Elk Point Group, sandstones and mudstones of the Lower Cretaceous Mannville Group, moderately lithified diamictites (probably Quaternary?) and unconsolidated Quaternary sediments. The Elk Point Group in this area can be further divided, from oldest to youngest, into the La Loche, Contact Rapids and Winnipegosis/Methy formations (Alberta nomenclature), or the Ashern and Winnipegosis formations (Saskatchewan nomenclature). The Mannville Group is divided, from lower to upper, into the McMurray, Clearwater and Grand Rapids formations (Alberta nomenclature), or the Cantuar and Pense formations (Saskatchewan nomenclature). -

Northern Saskatchewan Arts and Culture Handbook
Northern Saskatchewan Arts and Culture Handbook Compiled and edited by Miriam Körner for the Northern Sport, Culture and Recreation District Acknowledgements First and foremost I would like to thank the Northern Sport, Culture and Recreation District for contracting me to develop the Northern Saskatchewan Arts and Culture Handbook. Through this work I had the rare opportunity to visit many of Northern Saskatchewan’s finest arts and crafts people. I would like to thank all the artists that invited me into their homes, and shared their stories TheThe Northern Sport, Culture & with me. It was a wonderful experience and there is just one regret: That I did not have more RecreationRecreation DistrictDistrict wwouldould liklikee to time to spend with all of you! thankthank theirtheir sponsors:sponsors: This project would not have been possible without the outstanding support through dedicated individuals that helped me along my journey through northern communities. I especially would like to thank Rose Campbell & the Ospwakun Sepe School in Brabant; Simon Bird and Mary Thomas & the Reindeer Lake School in Southend; Marlene Naldzil & the Fond du Lac Denesuline First Nation Band Office, Rita Adam & the Father Gamache Memorial School, Flora Augier for room and board; Raymond McDonald and Corinne Sayazie & the Black Lake Denesuline Nation Band Office with special thanks to Mary Jane Cook for a great job finding artists, translating and lots of fun! Thanks to the Peter Ballantyne Cree Nation Band Office in Pelican Narrows and special thanks to Georgina Linklater for driving as well as Sheldon Dorion and Gertie Dorion for translating. Too bad I couldn’t stay longer! In Sandy Bay I would like to thank Lorraine Bear & the Hector Thiboutot Community School.