Transition Metal Complexes with Functionalized Silyl-Substituted Cyclopentadienyl and Related Ligands: Synthesis and Reactivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EI-ICHI NEGISHI Herbert C
MAGICAL POWER OF TRANSITION METALS: PAST, PRESENT, AND FUTURE Nobel Lecture, December 8, 2010 by EI-ICHI NEGISHI Herbert C. Brown Laboratories of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907-2084, U.S.A. Not long ago, the primary goal of the synthesis of complex natural products and related compounds of biological and medicinal interest was to be able to synthesize them, preferably before anyone else. While this still remains a very important goal, a number of today’s top-notch synthetic chemists must feel and even think that, given ample resources and time, they are capable of synthesizing virtually all natural products and many analogues thereof. Accepting this notion, what would then be the major goals of organic synthesis in the twenty-first century? One thing appears to be unmistakably certain. Namely, we will always need, perhaps increasingly so with time, the uniquely creative field of synthetic organic and organometallic chemistry to prepare both new and existing organic compounds for the benefit and well-being of mankind. It then seems reasonably clear that, in addition to the question of what compounds to synthesize, that of how best to synthesize them will become increasingly important. As some may have said, the primary goal would then shift from aiming to be the first to synthesize a given compound to seeking its ultimately satisfactory or “last synthesis”. If one carefully goes over various aspects of organic synthetic methodology, one would soon note how primitive and limited it had been until rather recently, or perhaps even today. For the sake of argument, we may propose here that the ultimate goal of organic synthesis is “to be able to synthesize any desired and fundamentally synthesizable organic compounds (a) in high yields, (b) efficiently (in as few steps as possible, for example), (c) selectively, preferably all in t98–99% selectivity, (d) economically, and (e) safely, abbreviated hereafter as the y(es)2 manner.” with or without catalyst R1M + R2X R1R2 + MX R1, R2: carbon groups. -

Catalytic Systems Based on Cp2zrx2 (X = Cl, H), Organoaluminum
catalysts Article Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization Lyudmila V. Parfenova 1,* , Pavel V. Kovyazin 1, Almira Kh. Bikmeeva 1 and Eldar R. Palatov 2 1 Institute of Petrochemistry and Catalysis of Russian Academy of Sciences, Prospekt Oktyabrya, 141, 450075 Ufa, Russia; [email protected] (P.V.K.); [email protected] (A.K.B.) 2 Bashkir State University, st. Zaki Validi, 32, 450076 Ufa, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-347-284-3527 i i Abstract: The activity and chemoselectivity of the Cp2ZrCl2-XAlBu 2 (X = H, Bu ) and [Cp2ZrH2]2- ClAlEt2 catalytic systems activated by (Ph3C)[B(C6F5)4] or B(C6F5)3 were studied in reactions with 1-hexene. The activation of the systems by B(C6F5)3 resulted in the selective formation of head- to-tail alkene dimers in up to 93% yields. NMR studies of the reactions of Zr complexes with organoaluminum compounds (OACs) and boron activators showed the formation of Zr,Zr- and Zr,Al-hydride intermediates, for which diffusion coefficients, hydrodynamic radii, and volumes were estimated using the diffusion ordered spectroscopy DOSY. Bis-zirconium hydride clusters of type x[Cp ZrH ·Cp ZrHCl·ClAlR ]·yRnAl(C F ) − were found to be the key intermediates of alkene 2 2 2 2 6 5 3 n dimerization, whereas cationic Zr,Al-hydrides led to the formation of oligomers. Citation: Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. -

Organometrallic Chemistry
CHE 425: ORGANOMETALLIC CHEMISTRY SOURCE: OPEN ACCESS FROM INTERNET; Striver and Atkins Inorganic Chemistry Lecturer: Prof. O. G. Adeyemi ORGANOMETALLIC CHEMISTRY Definitions: Organometallic compounds are compounds that possess one or more metal-carbon bond. The bond must be “ionic or covalent, localized or delocalized between one or more carbon atoms of an organic group or molecule and a transition, lanthanide, actinide, or main group metal atom.” Organometallic chemistry is often described as a bridge between organic and inorganic chemistry. Organometallic compounds are very important in the chemical industry, as a number of them are used as industrial catalysts and as a route to synthesizing drugs that would not have been possible using purely organic synthetic routes. Coordinative unsaturation is a term used to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Coordinative unsaturation is a very important concept in organotrasition metal chemistry. Hapticity of a ligand is the number of atoms that are directly bonded to the metal centre. Hapticity is denoted with a Greek letter η (eta) and the number of bonds a ligand has with a metal centre is indicated as a superscript, thus η1, η2, η3, ηn for hapticity 1, 2, 3, and n respectively. Bridging ligands are normally preceded by μ, with a subscript to indicate the number of metal centres it bridges, e.g. μ2–CO for a CO that bridges two metal centres. Ambidentate ligands are polydentate ligands that can coordinate to the metal centre through one or more atoms. – – – For example CN can coordinate via C or N; SCN via S or N; NO2 via N or N. -

A Novel Series of Titanocene Dichloride Derivatives: Synthesis, Characterization and Assessment of Their
A novel series of titanocene dichloride derivatives: synthesis, characterization and assessment of their cytotoxic properties by Gregory David Potter A thesis submitted to the Department of Chemistry in conformity with the requirements for the degree of Doctor of Philosophy Queen’s University Kingston, Ontario, Canada May, 2008 Copyright © Gregory David Potter, 2008 Abstract Although cis-PtCl2(NH3)2 (cisplatin) has been widely used as a chemotherapeutic agent, its use can be accompanied by toxic side effects and the development of drug resistance. Consequently, much research has been focused on the discovery of novel transition metal compounds which elicit elevated cytotoxicities coupled with reduced toxic side effects and non-cross resistance. Recently, research in this lab has focused on preparing derivatives of titanocene dichloride (TDC), a highly active chemotherapeutic agent, with pendant alkylammonium groups on one or both rings. Earlier results have demonstrated that derivatives containing either cyclic or chiral alkylammonium groups had increased cytotoxic activities. This research therefore investigated a new series of TDC complexes focusing specifically on derivatives bearing cyclic and chiral alkylammonium groups. A library of ten cyclic derivatives and six chiral derivatives were synthesized and fully characterized. These derivatives have undergone in vitro testing as anti-tumour agents using human lung, ovarian, and cervical carcinoma cell lines (A549, H209, H69, H69/CP, A2780, A2780/CP and HeLa). These standard cell lines represent solid tumour types for which new drugs are urgently needed. The potencies of all of the Ti (IV) derivatives varied greatly (range from 10.8 μM - >1000 μM), although some trends were observed. In general, the dicationic analogues exhibited greater potency than the corresponding monocationic derivatives. -

Cyclopentadienyl Compounds of the First Row Transition Metals And
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2017 Cyclopentadienyl Compounds of the First Row Transition Metals and Early Actinides: Novel Main-Group Bond Forming Catalysis and New Metallacycles Justin Kane Pagano University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Chemistry Commons Recommended Citation Pagano, Justin Kane, "Cyclopentadienyl Compounds of the First Row Transition Metals and Early Actinides: Novel Main-Group Bond Forming Catalysis and New Metallacycles" (2017). Graduate College Dissertations and Theses. 700. https://scholarworks.uvm.edu/graddis/700 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. CYCLOPENTADIENYL COMPOUNDS OF THE FIRST ROW TRANSITION METALS AND EARLY ACTINIDES: NOVEL MAIN-GROUP BOND FORMING CATALYSIS AND NEW METALLACYCLES A Dissertation Presented by Justin Kane Pagano to The Faculty of the Graduate College of The University of Vermont In Partial Fulfilment of the Requirements For the Degree of Doctor of Philosophy Specializing in Chemistry May, 2017 Defense Date: November 29, 2016 Dissertation Examination Committee: Rory Waterman, Ph. D., Advisor John M. Hughes, Ph. D., Chairperson Matthias Brewer, Ph. D. Jaqueline L. Kiplinger, Ph. D. Matthew D. Liptak, Ph. D. Cynthia J. Forehand, Ph. D., Dean of the Graduate College ABSTRACT Cyclopentadienyl first row transition-metal compounds have been well studied 5 since the 1950’s, with the nearly ubiquitous CpFe(CO)2Me (FpMe) (Cp = η -C5H5) being one of the first organometallics to be fully characterized. -

Synthesis and Reactivity of Cyclopentadienyl Based Organometallic Compounds and Their Electrochemical and Biological Properties
Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Sasmita Mishra Department of Chemistry National Institute of Technology Rourkela Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Dissertation submitted to the National Institute of Technology Rourkela In partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry by Sasmita Mishra (Roll Number: 511CY604) Under the supervision of Prof. Saurav Chatterjee February, 2017 Department of Chemistry National Institute of Technology Rourkela Department of Chemistry National Institute of Technology Rourkela Certificate of Examination Roll Number: 511CY604 Name: Sasmita Mishra Title of Dissertation: ''Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties We the below signed, after checking the dissertation mentioned above and the official record book(s) of the student, hereby state our approval of the dissertation submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry at National Institute of Technology Rourkela. We are satisfied with the volume, quality, correctness, and originality of the work. --------------------------- Prof. Saurav Chatterjee Principal Supervisor --------------------------- --------------------------- Prof. A. Sahoo. Prof. G. Hota Member (DSC) Member (DSC) --------------------------- -

Accepted Version
Citation for published version: Hamilton, JA, Pugh, T, Johnson, AL, Kingsley, AJ & Richards, SP 2016, 'Cobalt(I) olefin complexes: precursors for metal-organic chemical vapor deposition of high purity cobalt metal thin films', Inorganic Chemistry, vol. 55, no. 14, pp. 7141-7151. https://doi.org/10.1021/acs.inorgchem.6b01146, https://doi.org/10.1021/acs.inorgchem.6b01146 DOI: 10.1021/acs.inorgchem.6b01146 http://dx.doi.org/10.1021/acs.inorgchem.6b01146 Publication date: 2016 Document Version Peer reviewed version Link to publication This document is the Accepted Manuscript version of a Published Work that appeared in final form in Inorganic Chemistry, copyright © American Chemical Society after peer review and technical editing by the publisher. To access the final edited and published work see DOI: 10.1021/acs.inorgchem.6b01146. University of Bath Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 06. Oct. 2021 Table of Content Abstract and Graphic We report the synthesis and characterization of a family of eleven cobalt (I) metal precursors based around cyclopentadienyl and diene ligands. Low pressure metal-organic chemical vapor deposition (AP-MOCVD) was employed using the precursor cyclopentadienyl-cobalt(I)(isoprene) 1, to synthesis thin films of metallic cobalt on silicon substrates under an atmosphere (760 Torr) of hydrogen (H2) . -

US 2004/0237384 A1 Orr (43) Pub
US 2004O237384A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0237384 A1 Orr (43) Pub. Date: Dec. 2, 2004 (54) FUEL COMPOSITIONS EXHIBITING (52) U.S. Cl. ................. 44/314; 44/320; 44/358; 44/359; IMPROVED FUEL STABILITY 44/360; 44/444 (76) Inventor: William C. Orr, Denver, CO (US) Correspondence Address: (57)57 ABSTRACT HOGAN & HARTSON LLP ONE TABOR CENTER, SUITE 1500 A fuel composition of the present invention exhibits mini 1200 SEVENTEENTH ST mized hydrolysis and increased fuel Stability, even after DENVER, CO 80202 (US) extended storage at 65 F. for 6–9 months. The composition, which is preferably not strongly alkaline (3.0 to 10.5), is (21) Appl. No.: 10/722,063 more preferably weakly alkaline to mildly acidic (4.5 to 8.5) (22) Filed: Nov. 24, 2003 and most preferably slightly acidic (6.3 to 6.8), includes a e ars lower dialkyl carbonate, a combustion improving amount of Related U.S. Application Data at least one high heating combustible compound containing at least one element Selected from the group consisting of (63) Continuation-in-part of application No. 08/986,891, aluminum, boron, bromine, bismuth, beryllium, calcium, filed on Dec. 8, 1997, now Pat. No. 6,652,608. cesium, chromium, cobalt, copper, francium, gallium, ger manium, iodine, iron, indium, lithium, magnesium, manga Publication Classification nese, molybdenum, nickel, niobium, nitrogen, phosphorus, potassium, palladium, rubidium, Sodium, tin, Zinc, (51) Int. Cl." ........ C10L 1/12; C1OL 1/30; C1OL 1/28; praseodymium, rhenium, Silicon, Vanadium, or mixture, and C1OL 1/18 a hydrocarbon base fuel. -

Relative Chalcogen Donor Properties in Transition Metal Complexes. Eugene D
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1971 Relative Chalcogen Donor Properties in Transition Metal Complexes. Eugene D. Schermer Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Schermer, Eugene D., "Relative Chalcogen Donor Properties in Transition Metal Complexes." (1971). LSU Historical Dissertations and Theses. 1949. https://digitalcommons.lsu.edu/gradschool_disstheses/1949 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. 71-20,621 SCHERMER, Eugene D., 1934- RELATIVE CHALCOGEN DONOR PROPERTIES IN TRANSITION METAL COMPLEXES. The Louisiana State University and Agricultural and Mechanical College, Ph.D., 1971 Chemistry, inorganic University Microfilms, A XEROXCompany, Ann Arbor, Michigan THIS DISSERTATION HAS BEEN MICROFILMED EXACTLY AS RECEIVED Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. RELATIVE CHALCOGEN DONOR PROPERTIES IN TRANSITION METAL COMPLEXES A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Eugene D. Schemer B.A., Eastern Washington State College, 1958 M.S., Oregon State University, 1Q62 January, 197! Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGEMENT The author wishes to express his sincere gratitude to Professor William H. Baddley, under whose direction this work was produced. -

XXI. Syntheses of Etheno-Bridged Ansa-Titanocene Derivatives by The
207 ansa-Metallocene derivatives XXI *. Syntheses of etheno-bridged ansa-titanocene derivatives by the McMurry reaction Peter Burger and Hans H. Brintzinger * Fakultiit fur Chemie, Universitiit Konstanz, Postfach 5560, W-7750 Konstanz (Germany) Abstract Novel olefin-linked biscylopentadienylligands have been prepared by McMurry coupling reactions of benzoyl- and acetyl-cyclopentadienyl sodium. Reactions of the disodium salts with TiC1 4 or TiCl 3 • 3THF afford the alkene-bridged ansa-titanocene dichlorides. 13C-NMR shifts of the bridge carbon atoms do not indicate any interaction between the olefin 'IT-system and the metal. Introduction Chiral ansa-metallocenes of Group IV metals (of current interest as Ziegler-Natta catalysts for stereospecific a-olefin polymerisation [2-5]) with various types of interannular bridges X (a-f) (Fig. 1) have been synthesized. ansa-Metallocenes with long and flexible bridges, i.e. a and d, possess low stereorigidity. Increased distances between the bridge-head carbon atoms due to steric requirements of the bridge-atoms give rise to larger centroid-metal-centroid angles than in the corresponding un bridged metallocenes [4,8,9]. Dimethylsilyl- (c) and methylene- (f) linked biscyclo pentadienyl metal dichlorides, on the other hand, are very stereorigid but rather strained [4,6,7,14,15]. The geometry of ethano-bridged metallocenes (e) is almost identical to that of their unbridged congeners. The cyclopentadienyl (Cp) rings are staggered owing to an inclination of the C-C bond of the ethano bridge relative to the CI-M-Cl bisector plane. This gives rise to two enantiomeric forms, l) and A, both of which have been characterized by X-ray crystallographic studies [13,16,17]. -

US20100160506A1.Pdf
US 2010.0160506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0160506 A1 Wu et al. (43) Pub. Date: Jun. 24, 2010 (54) PRODUCTION OFSYNTHETIC Publication Classification HYDROCARBON FLUIDS, PLASTICIZERS (51) Int. Cl. AND SYNTHETICLUBRICANT BASE CSK 5/55 (2006.01) STOCKS FROM RENEWABLE FEEDSTOCKS CSK 5/109 (2006.01) C07D 30/03 (2006.01) (76) Inventors: Margaret May-Som Wu, Skillman, C07D 31 7/24 (2006.01) NJ (US); Karla Schall Colle, C07C I/207 (2006.01) Houston, TX (US); Ramzi Yanni (52) U.S. Cl. ......... 524/114; 524/280; 549/523: 549/229; Saleh, Baton Rouge, LA (US); 585/327 Allen D. Godwin, Seabrook, TX (57) ABSTRACT (US); John Edmond Randolph This disclosure is directed to an integrated method for making Stanat, Houston, TX (US) synthetic hydrocarbon fluids, plasticizers and polar synthetic lubricant base stocks from a renewable feedstock. More par Correspondence Address: ticularly, the disclosure is directed to a metathesis reaction of ExxonMobil Research & Engineering Company natural oil or its derivative ester and ethylene in the presence P.O. Box 900, 1545 Route 22 East of an effective amount of a metathesis catalyst to form linear Annandale, NJ 08801-0900 (US) alpha-olefins, internal olefins and reduced chain length trig lycerides. The linear alpha-olefins and/or internal olefins are polymerized to produce synthetic hydrocarbon fluids in the (21) Appl. No.: 12/633,742 presence of a suitable catalyst. The reduced chain length triglycerides are converted into polar synthetic lubricant base (22) Filed: Dec. 17, 2009 stocks or plasticizers by hydrogenation, isomerization, fol lowed by hydrogenations, or by hydroisomerization pro cesses. -
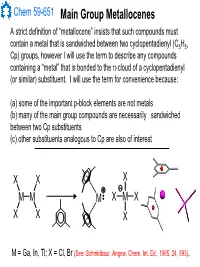
Main Group Metallocenes
Chem 59-651 Main Group Metallocenes A strict definition of “metallocene” insists that such compounds must contain a metal that is sandwiched between two cyclopentadienyl (C5H5, Cp) groups, however I will use the term to describe any compounds containing a “metal” that is bonded to the π-cloud of a cyclopentadienyl (or similar) substituent. I will use the term for convenience because: (a) some of the important p-block elements are not metals (b) many of the main group compounds are necessarily sandwiched between two Cp substituents (c) other substituents analogous to Cp are also of interest XX X MM M XMX X X X M = Ga, In, Tl; X = Cl, Br (See: Schmidbaur, Angew. Chem. Int. Ed., 1985, 24, 893). Chem 59-651 Cyclopentadienyl Ligand Basics The structural features and bonding interactions between cyclopentadienyl groups and metal atoms shows considerable variation and is described using a few different conventions (See Jutzi and Burford, Chem. Rev., 1999, 99, 969 and references therein). In most main group metallocenes, even those that are σ-bonded in the solid state, the rings rotate rapidly through a series of 1,2-shifts. A Cp group can be considered to be p-bonded to an element if the Hapticity: element sits inside the cylinder defined by the 5 carbon atoms of M MM the Cp ligand. The center of mass of the 5 C atoms is known as the centroid. η1-Cp η2-Cp η3-Cp M M M A σ-bonded Cp can be identified η4-Cp η5-Cp ≡η5-Cp by the angle at the ipso carbon M and bond lengths should indicate single bonds to the ipso carbon and a localized diene structure for σ the α and β carbon fragment.