United States Patent (11) 3,607,948 72) Inventor Donald Eldon Welton 2,855,437 10/1958 Lyons...... 260/610 A Victoria, Tex. 2,997,483 8/1961 Gray...... 260/617M (21) Appl. No. 706,018 3,238,238 3/1966 McNamara et al...... 260/617 M 22 Filed Feb. 16, 1968 3,333,010 7/1967 Urbanek...... 260/617 M 45) Patented Sept. 21, 1971 3,351,635 1 1/1967 Kollar...... 260/617 73 Assignee E. I. du Pont de Nemours and Company 3,391,213 7/1968 Fetterly.... 260/632 CP Wilmington, Del. 3,391,214 7/1968 Fetterly. 260/632 C 3,467,720 9/1969 List et al...... 260/632 C FOREIGN PATENTS 54 PREPARATION OF CYCLODODECANOL 8 Claims, 1 Drawing Fig. 918,444 2/1963 Great Britain...... 260/617 M 52 U.S. Cl...... 260/617 C, Primary Examiner-Leon Zitver 23/139,252/467,260/349.5 L, 260/531 R, Assistant Examiner-Joseph E. Evans 260/586 A, 260/610 B, 260/666 P Attorney-Earl L. Handley 51 Int. Cl...... C07c35/02, CO7c5/28 50 Field of Search...... 260/617 M, ABSTRACT: A process for the preparation of a cyclic alcohol 632 C, 632 CB, 610 B, 617 R by air oxidation of a cycloaliphatic compound to a mixture containing 1-30 percent by weight cycloaliphatic hydroperox (56 References Cited ide, oxidizing cycloolefin by means of the cycloaliphatic UNITED STATES PATENTS hydroperoxide, hydrogenating the resulting product, and 2,615,921 10/1952 Dougherty et al...... 260/632 C recovering cyclic alcohol and unconverted cycloaliphatic 2,671,119 3/1954 Mertzweiller...... 260/638 compound for recycle.
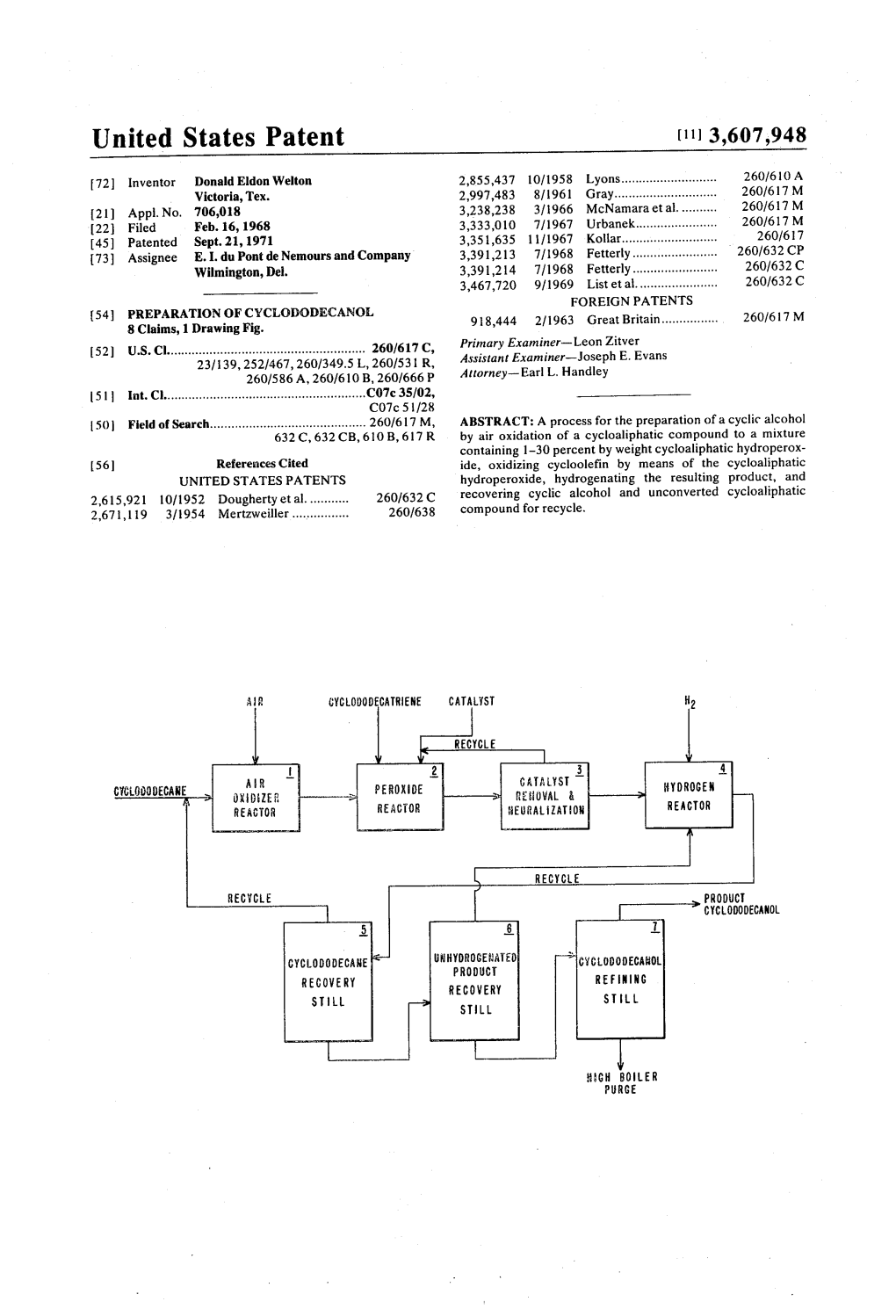
A. CYCOOODECATRENE CATALYST
RECYCLE
2 3 CATALYST T YDROGEN Crct.000DECANE J OE. PE ROKIDE RE i OVAL & REACTOR REACOR ERALIATON REACTOR
RECCE PRODUCT
CYC-000DECANOL
5 8 CYCLOODECASE were icy CLODODECAROL RECOVERY RECOVERY REFENING SL L
STLL STL
Ch BOLER PRCE PATENTED SEP21197 3,607.948
·3101038
3,607,948 1. 2 PREPARATION OF CYCLODODECANOL cyclododecatriene) present. The pressure of the reaction is not critical, and may vary from about 20 Torr to one or two at This invention relates to a process for the production of mospheres absolute. cycloaliphatic alcohols from cycloaliphatic compounds and The hydrogenation of the mixture containing the inter cycloolefins. 5 mediates is carried out using conventional hydrogenation It is known to air oxidize cyclododecane to a mixture con techniques, such as a fixed bed catalyst of nickel oxide, nickel taining cyclododecanol. The mixture also usually contains on Kieselguhr or alumina, supported platinum, and palladium other oxidative products, such as cyclododecanone and black; Raney nickel, cobalt molybdate, or nickel tungsten sul cyclododecyl hydroperoxide. Such air oxidation systems often fide. Such reactions are conventionally carried out at 100° C. include boric acid or another boron compound-see British 10 to 250 C., and at pressures of 40 to 5000 p.s. i., preferably 50 Pat. No. 1,032,390 to E. I. du Pont de Nemours and Company to 500 p.s. i., the particular conditions being selected so as to and British Pat NO. 1,064,167 to Esso Research & Engineer substantially completely saturate the olefinic unsaturation and ing Company. to reduce ketone to alcohol. A convenient source of cyclododecane is the trimerization of butadiene, followed by hydrogenation of this product. A 15 The attached schematic drawing further illustrates the convenient source of cyclooctane is dimerization ofbutadiene process. Cyclododecane and air are fed into an air oxidizer followed by hydrogenation of this product. reactor 1, wherein the cyclododecane is oxidized to a product The present invention is based on the discovery that the containing cyclododecyl hydroperoxide. This product is fed to product of any air oxidation system of cyclododecane which peroxide reactor 2 where it is mixed and reacted with contains 1 to 30 percent cyclododecyl hydroperoxide can be 20 cyclododecatriene in the presence of added catalyst. The used as a source of oxygen to convert cyclododecatriene to in reaction mixture is then fed to catalyst removal and neutral termediates, cyclododecatriene oxides, that upon hydrogena izer where it is suitably treated to remove the catalyst and tion gives cyclododecanol in high yields with only a small neutralize any organic acids formed by the oxidation steps, amount of byproduct. The cyclododecyl hydroperoxide is and any acidic catalyst residues that are present. The particu reduced upon reaction with the cyclododecatriene and it also 25 lar catalyst removal step carried out will depend on the forms cyclododecanol. This ability of the cyclododecyl catalyst employed. If boric acid is the catalyst, mere water hydroperoxide to oxidize cyclododecatriene is employed in wash at a temperature above about 60° C. will remove a the overall process as follows: cyclododecane is air oxidized to majority of the catalyst; the remainder is neutralized by the a mixture containing 1 to 30 percent cyclododecyl hydropero subsequent addition of mild alkali solution-pH of 8-12. The zide. This mixture will also contain small amounts of 30 boric acid is then dehydrated and recycled to the peroxide cyclododecanol, cyclododecanone, and other oxidative reactor. If the catalyst is not boric acid, but instead molybdic products. The cyclododecanol does not chemically react in oxide or the like, the catalyst may be removed by a water wash the remaining steps. Cyclododecatriene is then added to this that contains small amounts of alkali-pH of 8-12 at contact mixture in more than stoichiometric amounts, based upon the times of 1-30 minutes at temperatures of 60-1 10° C. Such amount of hydroperoxide present, usually in the amount of 1.1 35 catalysts may be discarded or purified and recycled. to three times the amount of hydroperoxide present, and the The reaction mixture is then passed into hydrogen reactor cyclododecatriene oxidized, forming oxygenated, olefinic in 4, where in the presence of a hydrogenation catalyst most of termediates. The mixture is then hydrogenated and the inter the cyclododecatriene oxides and cyclododecanone are mediates, cyclododecatriene oxides, are converted to 40 reduced to cyclododecanol, and cyclododecatriene which was cyclododecanol. The unreacted cyclododecatriene which was added in excess in the peroxide reaction is reduced to added in an excess amount is hydrogenated to cyclododecane. cyclododecane. The reaction mass is then passed to the The hydrogenation reaction also reduces the cyclododecane recovery still 5, where cyclododecane is cyclododecanone to cyclododecanol. The cyclododecanol is recovered and recycled to the air oxidation step. The remain then recovered, for example, by distillation and the 45 ing portion of the reaction mass is then passed to the un cyclododecane is recycled to the air oxidation step. hydrogenated product recovery still 6, where products that The air oxidation of cyclododecane to a mixture containing were not fully reduced to cyclododecane or cyclododecanoi cyclododecyl hydroperoxide may be carried out by any of the are removed and recycled to the hydrogenation reactor 4. several processes known in the art, either with or without a Finally, the reaction mass is passed into the cyclododecanol modifier such as boric acid. The preferred conditions are 50 refining still 7, where this product is removed from the high those which maximize hydroperoxide formation, Suitable con boiling organics formed in the previous reactions. ditions for bubbling air though cyclododecane are 140-170 It is possible to modify the reaction scheme in various ways, C., preferably as close to 140 C. as possible, and at at for example, more than one hydrogen reactor 4 could be em mospheric pressure. Ozone may be added to the air to allow ployed or long holdup times employed in a single reactor 4, use of lower temperatures. 55 thus eliminating the need for an unhydrogenated product The mixture containing the cyclododecyl hydroperoxide is recovery still 6. then reacted with cyclododecatriene. This latter reaction is In the following examples which illustrate the invention all preferably carried our at a temperature in the range of about parts and percentages are in parts by weight unless otherwise 60° C. to about 140° C. as below this temperature the mixture specified. is solid, and above this temperature the reaction goes with low 60 yield, preferably at 80° C. to 120° C.; a catalyst is also EXAMPLE preferably present. Suitable catalysts include boric acid com pounds such as orthoboric acid, boric oxide, borate esters, i.e. Cyclododecane was air oxidized in a conventional manner trimethyl borate, triethyl borate, vanadium compounds, such to form a mixture containing about 5.1 percent cyclododecyl as vanadium pentoxide, borovanadic acids, and oxides; molyb 65 hydroperoxide. 2.1 mols of cyclododecatriene and 1.8 mol of denum compounds such as molybdic anhydride, molybdic metaboric acid were added for each mol of hydroperoxide, acid, boromolybdic acids, peroxymolybdic acid, molybdenum and the mixture heated at 120° C. for 1.75 hours. 99 percent blue, molybdous oxide, and mixtures of these materials. When of the hydroperoxide was reacted by the end of this time. 0.49 the catalyst is boric acid, or a derivative, the amount added mol boric acid/mol of cyclododecyl hydroperoxide charged will be in the range of about 0.5 to 5 mol per mol of 70 went into solution. The boric acid was removed by hydrolysis cyclododecyl hydroperoxide present. When the catalyst is one and extraction with hot water. Upon analysis of the product it of the others mentioned, it will be about 0.005 percent to 0.5 was found that 0.85 mols of cyclododecatriene had been ox percent by weight of the total organics (cyclododecane, idized for each mol of cyclododecyl hydroperoxide present. cyclododecanol, cyclododecanone, cyclododecyl hydroperox The product was hydrogenated by adding 1 percent by weight ide, and other oxidation products and the added 75 palladium black catalyst, and pressured with hydrogen at 40 3,607,948 3 4 p.s. i. while it was agitated and heated to about 100° C. until cyclododecanone and 2.5 weight percent cyclododecane. This the pressure remained constant. Analysis of the product product is of sufficiently high purity to be used in this form, showed that 95 percent of the oxidized cyclododecatriene was but further distillation to further purify the product can be converted to cyclododecanol or products that on further carried out. (4) A high boiling residue containing about 0.88 hydrogenation would produce cyclododecanol; a total of 0.73 5 weight percent of the charge remained. This fraction con mol of these products was obtained for each mol of peroxide tained about 5 weight percent cyclododecanol. charged. The process illustrated by this invention can also be em ployed to make alcohols of other cyclic olefins, for example, EXAMPLE 2 the butadiene cyclic dimer could be used for the alcohol, 10 which by further oxidation will produce octane dioic acid. Cyclododecane was air oxidized to form a mixture contain I claim: ing 6.9 percent cyclododecyl hydroperoxide. 1. A process for the preparation of cyclododecanol which Cyclododecatriene was added in the amount of 2.1 mols/mol comprises the steps of (1) air oxidizing cyclododecane to form of hydroperoxide, and about 500 p.p.m. permolybdic oxide a mixture containing cyclododecanol, cyclododecanone, and catalyst was added and the temperature maintained at 100° C. 1 percent to 30 percent by weight cyclododecyl hydroperox for 1 hour. (The catalyst was prepared by suspending 1 part 15 ide, by bubbling air through cyclododecane at 140°C. to 170 molybdic anhydride (MoC)a) in 20 parts water and adding 5 C., (2) oxidizing cyclododecatriene by reacting it with said parts of a 30 percent solution of hydrogen peroxide. The solu mixture in the presence of a catalyst selected from the class tion was heated to 90-100° C. until a clear yellow solution was consisting of orthoboric acid, boric oxide, trimethyl borate, obtained, and then dried on a steam bath. The yellow solid 20 triethyl borate, vanadium pentoxide, borovanadic acid, analyzed, by iodometric analysis, 81 percent H.MoOs). At the vanadium oxide, molybdic anhydrode, molybdic acid, end of 1 hour the hydroperoxide was 98 percent reduced. The boromolybdic acid, peroxymolybdic acid, molybdenum blue, product was filtered. Analysis of the filtered product showed and molybdous oxide at a temperature of 60° C. to 140 C., that 0.80 mol of cyclododecatriene per mol of cyclododecyl the mol ratio to cyclododecatriene to cyclododecyl hydroperoxide was converted to products that on reduction 25 hydroperoxide being about 1.5 to 3, to form a mixture con would produce cyclododecanol. The product was washed with taining cyclododecatriene oxides, (3) hydrogenating this mix water containing a small amount of sodium hydroxide to ture using a hydrogenation catalyst selected from the class remove the catalyst and neutralize the organic acids and consisting of nickel oxide, nickel on Kieselguhr, nickel on alu catalyst residues. mina, supported platinum, palladium black, Raney nickel, The product was then mixed with about 2.5 percent by 30 cobalt molybdate, and nickel tungsten sulfide, at a tempera weight Raney nickel power and hydrogenated at 40-50 p.s.i.g. ture of 100° C.-250° C. and at a pressure of 40 to 5,000 at 100° C. until absorption of hydrogen ceased. The hydrogen pounds per square inch, (4) recovering cyclododecanol from absorbed 100 percent of that calculated from analysis of the this hydrogenated product. feed to be necessary to convert all unreacted 2. The process of claim 1 in which the catalyst used in the cyclododecatriene to cyclododecane and all the oxygenated 35 oxidizing of the cyclododecatriene is removed prior to product to cyclododecanol. The catalyst was removed by fil hydrogenating the mixture containing cyclododecatriene ox tration and the product analyzed. It contained 10.5 weight ides. percent cyclododecanol, 0.27 weight percent 3. The process of claim 2 in which the cyclododecane is cyclododecanone, and 0.04 percent cyclododecyl epoxide. recovered from the hydrogenated product and recycled to the The product was subjected to vacuum distillation and 40 air oxidation step. separated into four fractions to give 99.1 weight percent 4. The process of claim 3 in which a mixture containing recovery. (1) A fraction was removed at 104-105 C. at a cyclododecanone and cyclododecane epoxide is recovered pressure of 10 torr. This fraction contained about 86.8 per from the hydrogenated product and recycled to the cent of the charged product, and contained mostly hydrogenation step. cyclododecane with about 0.3 weight percent cyclododecanol 45 5. The process of claim 4 in which the air oxidized product and cyclododecanone. This fraction may be recycled to the air is neutralized by washing with dilute aqueous alkali prior to oxidation step. (2) The pressure was then reduced to about 6 hydrogenation of the air oxidized product. torr and a fraction removed having a boiling point of 97-132 6. The process of claim 1 in which the catalyst employed in C. This fraction contained about 3.5 weight percent of the the oxidation of cyclododecatriene is orthoboric acid, and said charged product. This fraction contained about 54 weight per 50 catalyst is present in the amount of 0.5 to 5 mol per mol of cent cyclododecanol, 5.1 weight percent cyclododecanone, cyclododecyl hydroperoxide present. 0.4 weight percent cyclododecane epoxide and 43.4 weight 7. The process of claim 1 in which the catalyst employed in percent cyclododecane. This fraction may be recycled to the the oxidation of cyclododecatriene is a molybdenum contain hydrogenation step to convert the cyclododecane epoxide and ing catalyst and said catalyst is present in the amount of 0.005 cyclododecanone to cyclododecanol, (3) The temperature 55 to 0.5 percent by weight of the total organics present. was then increased while maintaining the pressure at about 6 8. The process of claim 1 in which the catalyst employed in torr and a fraction containing about 8.0 weight percent of the the oxidation of cyclododecatriene is a vanadium containing charged product was recovered. This fraction contained catalyst and said catalyst is present in the amount of 0.005 to greater than 96 weight percent cyclododecanol, 0.3 weight 0.5 percent by weight of the total organics present. percent cyclododecane epoxide, 1.0 weight percent 60
65
70
75