Pediatric Pathology Warm-‐Up Answers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Problems in Diagnosis Approach for Carcinoma of Pancreatic Head
CASE REPORT Problems in Diagnosis Approach for Carcinoma of Pancreatic Head Ratu Ratih Kusumayanti*, Marcellus Simadibrata**, Murdani Abdullah**, Rino Alvani Gani***, Lies Luthariana* *Department of Internal Medicine, Faculty of Medicine, University of Indonesia Dr. Cipto Mangunkusumo General National Hospital, Jakarta ** Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta *** Division of Hepatology, Department of Internal Medicine, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo General National Hospital, Jakarta ABSTRACT Incidences of pancreatic cancer worldwide have been known to be increased. It is the fifth leading cause of death in United State of America. Seventy percent occurs in the head of the pancreas. Major risk factors are related to age, black race, smokers, high-fat diet, chronic pancreatitis, diabetes mellitus and alcohol consumption. Some clinical symptoms such as jaundice, abdominal pain, unexplained weight loss or ascites can occur early or even late in the course of disease. Diagnosing pancreatic cancer sometimes can be difficult, regarding to discrepancy between clinical symptoms and radiological findings. It is important to take good history of the patient, thorough examination, and combine several modalities in diagnosing tumor of pancreatic head. In this case report, a 54 year-old female, came to the hospital with abdominal swelling and jaundice. Physical examination revealed liver and spleen enlargement and edema on both lower extremities. The laboratory result showed increment in Carcinoembryonic Antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) level, without marked increase in bilirubin level. Dilatation of the pancreatic duct was found in this patient, without any sign of bile stone. -

A Rare Presentation of Benign Brenner Tumor of Ovary: a Case Report
International Journal of Reproduction, Contraception, Obstetrics and Gynecology Periasamy S et al. Int J Reprod Contracept Obstet Gynecol. 2018 Jul;7(7):2971-2974 www.ijrcog.org pISSN 2320-1770 | eISSN 2320-1789 DOI: http://dx.doi.org/10.18203/2320-1770.ijrcog20182920 Case Report A rare presentation of benign Brenner tumor of ovary: a case report Sumathi Periasamy1, Subha Sivagami Sengodan2*, Devipriya1, Anbarasi Pandian2 1Department of Surgery, 2Department of Obstetrics and Gynaecology, Government Mohan Kumaramangalam Medical College, Salem, Tamil Nadu, India Received: 17 April 2018 Accepted: 23 May 2018 *Correspondence: Dr. Subha Sivagami Sengodan, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Brenner tumors are rare ovarian tumors accounting for 2-3% of all ovarian neoplasms and about 2% of these tumors are borderline (proliferating) or malignant. These tumors are commonly seen in 4th-8th decades of life with a peak in late 40s and early 50s. Benign Brenner tumors are usually small, <2cm in diameter and often detected incidentally during surgery or on pathological examination. Authors report a case of a large, calcified benign Brenner tumor in a 55-year-old postmenopausal woman who presented with complaint of abdominal pain and mass in abdomen. Imaging revealed large complex solid cystic pelvic mass -peritoneal fibrosarcoma. She underwent laparotomy which revealed huge Brenner tumor weighing 9kg arising from left uterine cornual end extending up to epigastric region. -

Case Report Metastasis of Pancreatic Cancer Within Primary Colon Cancer by Overtaking the Stromal Microenvironment
Int J Clin Exp Pathol 2018;11(6):3141-3146 www.ijcep.com /ISSN:1936-2625/IJCEP0075771 Case Report Metastasis of pancreatic cancer within primary colon cancer by overtaking the stromal microenvironment Takeo Nakaya1, Hisashi Oshiro1, Takumi Saito2, Yasunaru Sakuma2, Hisanaga Horie2, Naohiro Sata2, Akira Tanaka1 Departments of 1Pathology, 2Surgery, Jichi Medical University, Shimotsuke, Tochigi, Japan Received March 10, 2018; Accepted April 15, 2018; Epub June 1, 2018; Published June 15, 2018 Abstract: We report a unique case of a 74-old man, who presented with double cancers, showing metastasis of pancreatic cancer to colon cancer. Histopathological examination after surgery revealed that the patient had as- cending colon cancer, which metastasized to the liver (pT4N0M1), as well as pancreatic cancer (pT2N1M1) that metastasized to the most invasive portion of the colon cancer, namely the serosal to subserosal layers. Although the mechanisms for this scenario have yet to be elucidated, we speculate that the metastatic pancreatic carcinoma overtook the stromal microenvironment of the colon cancer. Namely, the cancer microenvironment enriched by can- cer-associated fibroblasts, which supported the colon cancer, might be suitable for the invasion and engraftment by pancreatic carcinoma. The similarity of histological appearance might make it difficult to distinguish metastatic pancreatic carcinoma within colon cancer. Furthermore, the metastasis of pancreatic carcinoma in colon carcinoma might be more common, despite it not having been previously reported. Keywords: Cancer metastasis, metastatic pancreatic cancer, colon cancer, double cancer, tumor microenviron- ment Introduction Case report Prevention and control of cancer metastasis is Clinical history one of the most important problems in cancer care [1-4]. -

Immunohistochemical and Electron Microscopic Findings in Benign Fibroepithelial Vaginal Polyps J Clin Pathol: First Published As 10.1136/Jcp.43.3.224 on 1 March 1990
224 J Clin Pathol 1990;43:224-229 Immunohistochemical and electron microscopic findings in benign fibroepithelial vaginal polyps J Clin Pathol: first published as 10.1136/jcp.43.3.224 on 1 March 1990. Downloaded from T P Rollason, P Byrne, A Williams Abstract LIGHT MICROSCOPY Eleven classic benign "fibroepithelial Sections were cut from routinely processed, polyps" of the vagina were examined paraffin wax embedded blocks at 4 gm and using a panel of immunocytochemical immunocytochemical techniques were agents. Two were also examined electron performed using a standard peroxidase- microscopically. In all cases the stellate antiperoxidase method.7 The antibodies used and multinucleate stromal cells were as follows: polyclonal rabbit characteristic of these lesions stained antimyoglobin (batch A324, Dako Ltd, High strongly for desmin, indicating muscle Wycombe, Buckinghamshire), monoclonal intermediate filament production. In anti-desmin (batch M724, Dako Ltd), mono- common with uterine fibroleiomyomata, clonal anti-epithelial membrane antigen (batch numerous mast cells were also often M613, Dako Ltd), monoclonal anti-vimentin seen. Myoglobin staining was negative. (batch M725, Dako Ltd), polyclonal rabbit Electron microscopical examination anti-cytokeratin (Bio-nuclear services, Read- confirmed that the stromal cells con- ing) and monoclonal anti cytokeratin NCL tained abundant thin filaments with focal 5D3 (batch M503, Bio-nuclear services). densities and also showed the ultrastruc- Mast cells were shown by a standard tural features usually associated with chloroacetate esterase method using pararo- myofibroblasts. saniline,8 which gave an intense red cyto- It is concluded that these tumours plasmic colouration, and by the routine would be better designated polypoid toluidine blue method. myofibroblastomas in view of the above An attempt was made to assess semiquan- findings. -

The Health-Related Quality of Life of Sarcoma Patients and Survivors In
Cancers 2020, 12 S1 of S7 Supplementary Materials The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of A Nationwide Observational Study (PROSa) Martin Eichler, Leopold Hentschel, Stephan Richter, Peter Hohenberger, Bernd Kasper, Dimosthenis Andreou, Daniel Pink, Jens Jakob, Susanne Singer, Robert Grützmann, Stephen Fung, Eva Wardelmann, Karin Arndt, Vitali Heidt, Christine Hofbauer, Marius Fried, Verena I. Gaidzik, Karl Verpoort, Marit Ahrens, Jürgen Weitz, Klaus-Dieter Schaser, Martin Bornhäuser, Jochen Schmitt, Markus K. Schuler and the PROSa study group Includes Entities We included sarcomas according to the following WHO classification. - Fletcher CDM, World Health Organization, International Agency for Research on Cancer, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. 468 p. (World Health Organization classification of tumours). - Kurman RJ, International Agency for Research on Cancer, World Health Organization, editors. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. 307 p. (World Health Organization classification of tumours). - Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur Urol. 2016 Jul;70(1):106–19. - World Health Organization, Swerdlow SH, International Agency for Research on Cancer, editors. WHO classification of tumours of haematopoietic and lymphoid tissues: [... reflects the views of a working group that convened for an Editorial and Consensus Conference at the International Agency for Research on Cancer (IARC), Lyon, October 25 - 27, 2007]. 4. ed. -

Rare Pancreatic Tumors
Published online: 2020-04-29 THIEME 64 ReviewRare Pancreatic Article Tumors Choudhari et al. Rare Pancreatic Tumors Amitkumar Choudhari1,2 Pooja Kembhavi1,2 Mukta Ramadwar3,4 Aparna Katdare1,2 Vasundhara Smriti1,2 Akshay D. Baheti1,2 1Department of Radiodiagnosis, Tata Memorial Hospital, Mumbai, Address for correspondence Akshay D. Baheti, MD, Department of Maharashtra, India Radiodiagnosis, Tata Memorial Hospital, Ernest , Borges Marg Parel 2Department of Radiodiagnosis, Homi Bhabha National University, Mumbai 400012, India (e-mail: [email protected]). Mumbai, Maharashtra, India 3Department of Pathology, Tata Memorial Hospital, Mumbai, Maharashtra, India 4Department of Pathology, Homi Bhabha National University, Mumbai, Maharashtra, India J Gastrointestinal Abdominal Radiol ISGAR 2020;3:64–74 Abstract Pancreatic ductal adenocarcinoma, neuroendocrine tumor, and cystic pancreatic neo- plasms are the common pancreatic tumors most radiologists are familiar with. In this Keywords article we review the clinical presentation, pathophysiology, and radiology of rare pan- ► pancreatic cancer creatic neoplasms. While the imaging features are usually nonspecific and diagnosis is ► uncommon based on pathology, the radiology along with patient demographics, history, and lab- ► pancreatoblastoma oratory parameters can often help indicate the diagnosis of an uncommon pancreatic ► acinar cell neoplasm and guide appropriate management in these cases. ► lymphoma Introduction hyperlipasemia may rarely lead to extraabdominal manifes- tations like ectopic subcutaneous fat necrosis and polyarthri- Pancreatic tumors of various histological subtypes can be tis (lipase hypersecretion syndrome).4 encountered in clinical practice, most common being pan- These tumors are hypoenhancing compared with the pan- creatic ductal adenocarcinoma (PDAC), which constitutes creas and are frequently associated with cystic or necrotic 85% of all pancreatic neoplasms.1 Histologically pancreat- areas as well as calcifications5,6 (►Fig. -

Fibro-Epithelial Polyp: Case Report with Literature Review
ISSN: 2456-8090 (online) CASE REPORT International Healthcare Research Journal 2017;1(7):14-17. DOI: 10.26440/IHRJ/01_07/116 QR CODE Fibro-Epithelial Polyp: Case Report with Literature Review RATNA SAMUDRAWAR 1, HEENA MAZHAR2, MUKESH KUMAR KASHYAP3, RUBI GUPTA4 A Oral fibroma is the most common benign soft tissue tumor caused due to continuous trauma from sharp cusp of teeth or faulty dental B restoration. It presents as sessile or occasionally pedunculated painless swelling which can be soft to firm in consistency. Its incidence occurs mostly during third to fifth decade and shows preference for female. Its occurrence corresponds with intraoral areas that are S prone to trauma such as the tongue, buccal mucosa and labial mucosa, lip, gingiva. Even with conservative surgical excision, the T lesion may recur until the source of continuous irritation persists. This article presents a case of large size oral fibroma on left alveolar R region associated with ulceration along with literature review. A C KEYWORDS: Benign Connective Tissue Tumor, Fibro-epithelial Polyp, Irritation Fibroma, Traumatic Fibroma, Focal Fibrous T Hyperplasia. K INTRODUCTION Fibroma of the oral mucosa is the most common complaint of growth in left lower back region of benign soft tissue tumor of the oral cavity derived the mouth since 4 months. History elicited that from fibrous connective tissues (CTs).1 Its the a solitary, painless growth had been observed pathogenesis lies in the fact that due to continues in his left mandibular molar region which was local trauma, a type of reactive hyperplasia of initially small in size and then it gradually fibrous tissue occurs.2 Thus, “Focal fibrous enlarged to present size of oval shape, well- hyperplasia” (FFH) term was suggested by Daley defined, pedunculated lesion. -
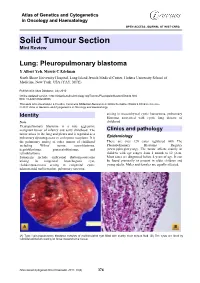
Solid Tumour Section Mini Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Solid Tumour Section Mini Review Lung: Pleuropulmonary blastoma Y Albert Yeh, Morris C Edelman North Shore University Hospital, Long Island Jewish Medical Center, Hofstra University School of Medicine, New York, USA (YAY, MCE) Published in Atlas Database: July 2010 Online updated version : http://AtlasGeneticsOncology.org/Tumors/PleuropulmblastomID6040.html DOI: 10.4267/2042/45006 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2011 Atlas of Genetics and Cytogenetics in Oncology and Haematology arising in mesenchymal cystic hamartoma, pulmonary Identity blastoma associated with cystic lung disease of Note childhood. Pleuropulmonary blastoma is a rare aggressive malignant tumor of infancy and early childhood. The Clinics and pathology tumor arises in the lung and pleura and is regarded as a pulmonary dysontogenetic or embryonic neoplasm. It is Epidemiology the pulmonary analog of other tumors of childhood There are over 120 cases registered with The including Wilms' tumor, neuroblastoma, Pleuropulmonary Blastoma Registry hepatoblastoma, pancreatoblastoma, and (www.ppbregistry.org). The tumor affects mainly in retinoblastoma. children with age ranges from 1 month to 12 years. Synonyms include embryonal rhabomyosarcoma Most cases are diagnosed before 4 years of age. It can arising in congenital bronchogenic cyst, be found prenatally or present in older children and rhabdomyosarcoma arising in congenital cystic young adults. Males and females are equally affected. adenomatoid malformation, pulmonary sarcoma (A) Type I pleuropulmonary blastoma consists of multiloculated cyst filled with scanty clear serous fluid. (B) The cysts are lined by cuboidal epithelium resting on loose mesenchymal tissue. -

Soft Tissue Sarcoma Classifications
Soft Tissue Sarcoma Classifications Contents: 1. Introduction 2. Summary of SSCRG’s decisions 3. Issue by issue summary of discussions A: List of codes to be included as Soft Tissue Sarcomas B: Full list of codes discussed with decisions C: Sarcomas of neither bone nor soft tissue D: Classifications by other organisations 1. Introduction We live in an age when it is increasingly important to have ‘key facts’ and ‘headline messages’. The national registry for bone and soft tissue sarcoma want to be able to produce high level factsheets for the general public with statements such as ‘There are 2000 soft tissue sarcomas annually in England’ or ‘Survival for soft tissue sarcomas is (eg) 75%’ It is not possible to write factsheets and data briefings like this, without a shared understanding from the SSCRG about which sarcomas we wish to include in our headline statistics. The registry accepts that soft tissue sarcomas are a very complex and heterogeneous group of cancers which do not easily reduce to headline figures. We will still strive to collect all data from cancer registries about anything that is ‘like a sarcoma’. We will also produce focussed data briefings on sites such as dermatofibrosarcomas and Kaposi’s sarcomas – the aim is not to forget any sites we exclude! The majority of soft tissue sarcomas have proved fairly uncontroversial in discussions with individual members of the SSCRG, but there were 7 particular issues it was necessary to make a group decision on. This paper records the decisions made and the rationale behind these decisions. 2. Summary of SSCRG’s decisions: Include all tumours with morphology codes as listed in Appendix A for any cancer site except C40 and C41 (bone). -

Transcriptional Regulation of IGF-I Receptor Gene Expression by Novel Isoforms of the EWS-WT1 Fusion Protein
Oncogene (2002) 21, 1890 ± 1898 ã 2002 Nature Publishing Group All rights reserved 0950 ± 9232/02 $25.00 www.nature.com/onc Transcriptional regulation of IGF-I receptor gene expression by novel isoforms of the EWS-WT1 fusion protein Ina Finkeltov1, Scott Kuhn3,4, Tova Glaser1, Gila Idelman1, John J Wright2, Charles T Roberts Jr3 and Haim Werner*,1 1Department of Clinical Biochemistry, Sackler School of Medicine, Tel Aviv University, Ramat Aviv, 69978 Israel; 2Medicine Branch, Division of Clinical Science, National Cancer Institute, NIH, Bethesda, Maryland MD 20889, USA; 3Department of Pediatrics, Oregon Health Sciences University, Portland, Oregon OR 97201, USA The EWS family of genes is involved in numerous Werner et al., 1994a). In addition to its central role in chromosomal translocations that are characteristic of a normal growth processes, the IGF-I-R plays a pivotal variety of sarcomas. A recently described member of this role in malignant transformation (Baserga et al., 1994; group is desmoplastic small round cell tumor (DSRCT), Grimberg and Cohen, 2000; Werner and LeRoith, which is characterized by a recurrent t(11;22)(p13;q12) 1996). The IGF-I-R is highly expressed in most tumors translocation that fuses the 5' exons of the EWS gene to and cancer cell lines, where it functions as a potent the 3' exons of the WT1 gene. The originally described antiapoptotic agent, conferring enhanced survival to chimera comprises exons 1 ± 7 of EWS and exons 8 ± 10 malignant cells (Resnico et al., 1995; Werner and of WT1. We have previously reported that the WT1 LeRoith, 1997). Transcription of the IGF-I-R gene is protein represses the expression of the IGF-I receptor negatively regulated by a number of tumor suppres- gene, whereas the EWS(1 ± 7)-WT1(8 ± 10) fusion protein sors, including p53, BRCA1 and WT1 (Maor et al., activates IGF-I receptor gene expression. -

About Soft Tissue Sarcoma Overview and Types
cancer.org | 1.800.227.2345 About Soft Tissue Sarcoma Overview and Types If you've been diagnosed with soft tissue sarcoma or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is a Soft Tissue Sarcoma? Research and Statistics See the latest estimates for new cases of soft tissue sarcoma and deaths in the US and what research is currently being done. ● Key Statistics for Soft Tissue Sarcomas ● What's New in Soft Tissue Sarcoma Research? What Is a Soft Tissue Sarcoma? Cancer starts when cells start to grow out of control. Cells in nearly any part of the body can become cancer and can spread to other areas. To learn more about how cancers start and spread, see What Is Cancer?1 There are many types of soft tissue tumors, and not all of them are cancerous. Many benign tumors are found in soft tissues. The word benign means they're not cancer. These tumors can't spread to other parts of the body. Some soft tissue tumors behave 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 in ways between a cancer and a non-cancer. These are called intermediate soft tissue tumors. When the word sarcoma is part of the name of a disease, it means the tumor is malignant (cancer).A sarcoma is a type of cancer that starts in tissues like bone or muscle. Bone and soft tissue sarcomas are the main types of sarcoma. Soft tissue sarcomas can develop in soft tissues like fat, muscle, nerves, fibrous tissues, blood vessels, or deep skin tissues. -

The Role of Cytogenetics and Molecular Diagnostics in the Diagnosis of Soft-Tissue Tumors Julia a Bridge
Modern Pathology (2014) 27, S80–S97 S80 & 2014 USCAP, Inc All rights reserved 0893-3952/14 $32.00 The role of cytogenetics and molecular diagnostics in the diagnosis of soft-tissue tumors Julia A Bridge Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, USA Soft-tissue sarcomas are rare, comprising o1% of all cancer diagnoses. Yet the diversity of histological subtypes is impressive with 4100 benign and malignant soft-tissue tumor entities defined. Not infrequently, these neoplasms exhibit overlapping clinicopathologic features posing significant challenges in rendering a definitive diagnosis and optimal therapy. Advances in cytogenetic and molecular science have led to the discovery of genetic events in soft- tissue tumors that have not only enriched our understanding of the underlying biology of these neoplasms but have also proven to be powerful diagnostic adjuncts and/or indicators of molecular targeted therapy. In particular, many soft-tissue tumors are characterized by recurrent chromosomal rearrangements that produce specific gene fusions. For pathologists, identification of these fusions as well as other characteristic mutational alterations aids in precise subclassification. This review will address known recurrent or tumor-specific genetic events in soft-tissue tumors and discuss the molecular approaches commonly used in clinical practice to identify them. Emphasis is placed on the role of molecular pathology in the management of soft-tissue tumors. Familiarity with these genetic events