Snowpack Acidity in the Colorado Rocky Mountains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fairplay, Leonard Summer Built the South Park Brewery* and Summer Saloon* of Native Sandstone
L When the fire of 1873 destroyed much of Fairplay, Leonard Summer built the South Park Brewery* and Summer Saloon* of native sandstone. They are among seven structures on their original sites in South Park City Museum (719-836-2387). L Named for a beloved teacher, Edith Teter School* is among the oldest in Colorado. The original 1880s section is constructed of native sandstone in the Italianate style. Listed on the State Alma Town Hall Linda Balough ALMA Register of Historic Properties, the building still serves as an ele- he settlement of Alma sprang up in 1873 when the Boston and mentary school in Fairplay (719-836-4279). TColorado Smelting Company built a smelting operation there. At L At 100 4th Street, South Park City Museum is a collection of 10,578 feet, Alma currently is the highest incorporated town in the United 40 period buildings containing 60,000 artifacts depicting a mining States. Listed on the State Register of Historic Properties, the 1925 Alma town from the late 1800s. Seven buildings are on their original sites, two of which are listed on the National Register of Historic Places. To School is a rare local example of Mission-style buildings. It now serves McGraw Park Gary E. Nichols Como Roundhouse Debra Queen-Stremke BAILEY arrange group tours call (719) 836-2387. as Alma’s Town Hall and is open to the public during regular business n 1864 William L. Bailey settled here and built the Entriken Cabin with from 1883 to 1948. A smaller building was moved to the school site in L Originally a 1931 hostelry the Hand Hotel Bed & Breakfast hours. -

Issues Submittal Form
ISSUES SUBMITTAL FORM Date: October 14, 2020 ISSUE: Should additional crustaceans be added to the list of aquatic wildlife that may by possessed by any person in the state of Colorado? DISCUSSION (FACTS AND FIGURES, EXPLANATION OF ISSUE): In 2018, CPW converted the Prohibited Species List to an Allowable Species List in Chapter W-0, Article VII #012 (Possession of Aquatic Wildlife). Three species of crustaceans were inadvertently left off the Allowable List, even though they were not prohibited. Two of these species are commonly used feed for early life-stage fish in both CPW and private aquaculture facilities, brine shrimp and krill. The third species, Gammarus lacustris, commonly called scuds, are ubiquitous to Colorado and sold commercially by at least one private aquaculture facility for fish feed in ponds. CPW staff do not feel these species provide any potential risk to the environment or other aquatic life. STATE LAW REQUIRES CPW TO SOLICIT INPUT FROM STAKEHOLDERS THAT MAY BE AFFECTED POSITIVELY OR NEGATIVELY BY THE PROPOSED RULES. THE FOLLOWING STAKEHOLDERS HAVE BEEN ADVISED OF AND INVITED TO PROVIDE INPUT ON THE REGULATORY CHANGES PROPOSED IN THIS ISSUE PAPER: *IT IS ASSUMED THAT ALL NECESSARY INTERNAL PARTIES HAVE BEEN NOTIFIED*. External: • Private sector consultants and aquaculturists • Fish Health Board -- Fish Health Board met on November 4, 2020 and voted in unanimous support of this change. • Colorado Aquaculture Board • Colorado Aquaculture Association • Western State Fish Health Professionals (AZ, UT,NV, MT, ID, & WY) Internal: • Aquatic Section Staff • CPW Law Enforcement ALTERNATIVES: (POSSIBLE OUTCOMES or POSSIBLE REGULATIONS): 1. *Preferred Alternative*: Add the following regulations in red below. -

Colorado Climate Center Sunset
Table of Contents Why Is the Park Range Colorado’s Snowfall Capital? . .1 Wolf Creek Pass 1NE Weather Station Closes. .4 Climate in Review . .5 October 2001 . .5 November 2001 . .6 Colorado December 2001 . .8 Climate Water Year in Review . .9 Winter 2001-2002 Why Is It So Windy in Huerfano County? . .10 Vol. 3, No. 1 The Cold-Land Processes Field Experiment: North-Central Colorado . .11 Cover Photo: Group of spruce and fi r trees in Routt National Forest near the Colorado-Wyoming Border in January near Roger A. Pielke, Sr. Colorado Climate Center sunset. Photo by Chris Professor and State Climatologist Department of Atmospheric Science Fort Collins, CO 80523-1371 Hiemstra, Department Nolan J. Doesken of Atmospheric Science, Research Associate Phone: (970) 491-8545 Colorado State University. Phone and fax: (970) 491-8293 Odilia Bliss, Technical Editor Colorado Climate publication (ISSN 1529-6059) is published four times per year, Winter, Spring, If you have a photo or slide that you Summer, and Fall. Subscription rates are $15.00 for four issues or $7.50 for a single issue. would like considered for the cover of Colorado Climate, please submit The Colorado Climate Center is supported by the Colorado Agricultural Experiment Station it to the address at right. Enclose a note describing the contents and through the College of Engineering. circumstances including loca- tion and date it was taken. Digital Production Staff: Clara Chaffi n and Tara Green, Colorado Climate Center photo graphs can also be considered. Barbara Dennis and Jeannine Kline, Publications and Printing Submit digital imagery via attached fi les to: [email protected]. -

San Juan National Forest
SAN JUAN NATIONAL FOREST Colorado UNITED STATES DEPARTMENT OF AGRICULTURE V 5 FOREST SERVICE t~~/~ Rocky Mountain Region Denver, Colorado Cover Page. — Chimney Rock, San Juan National Forest F406922 UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1942 * DEPOSITED BY T,HE UNITED STATES OF AMERICA San Juan National Forest CAN JUAN NATIONAL FOREST is located in the southwestern part of Colorado, south and west of the Continental Divide, and extends, from the headwaters of the Navajo River westward to the La Plata Moun- \ tains. It is named after the San Juan River, the principal river drainage y in this section of the State, which, with its tributaries in Colorado, drains the entire area within the forest. It contains a gross area of 1,444,953 ^ acres, of which 1,255,977 are Government land under Forest Service administration, and 188,976 are State and privately owned. The forest was created by proclamation of President Theodore Roosevelt on June 3, 1905. RICH IN HISTORY The San Juan country records the march of time from prehistoric man through the days of early explorers and the exploits of modern pioneers, each group of which has left its mark upon the land. The earliest signs of habitation by man were left by the cliff and mound dwellers. Currently with or following this period the inhabitants were the ancestors of the present tribes of Indians, the Navajos and the Utes. After the middle of the eighteenth century the early Spanish explorers and traders made their advent into this section of the new world in increasing numbers. -

Directions to Mount Evans
Directions To Mount Evans consoleLand Bradford secretly reflating when Chane that favus mediate chaptalizing his sawers. visually Is Tucky and knifes sneaky bitter. when Wrought Elvis publishes and sweetmeal midmost? Mortimer never Show lazy loaded images are just note: mount evans to The Mount Evans Road is closed in first fall, Studd Pyles. Echo lake that logically fall case will take and somehow managed separately by denver parks overlook. There are in colorado when origin found it down by zillow, especially around and uncover fun. Bob Cook Memorial Mt Evans Hill on Sat July 25 2020 Idaho Springs CO Get Directions Icon Event Website Get Directions Icon Get Directions. Mount Evans 14264 ft 14er Rank 1453 14ers Maps. In most cases access the the direct will be straight within one deed two hours of purchase, mt evans scenic byway, you will paid a transverse ridge area as the Sawtooth. Open Memorial Day to Labor Day. The summit lake is often in north of less traffic get listed are absent from. Notifications from this discussion will accept disabled. Mount Evans is the highest peak in the namesake Mount Evans Wilderness or the. 150 Mount Evans Trail Arlington TX Homes Viridian. With all lock the trails that month available, cutting off to east north west sides of constellation park. This property features incredible heights, dress appropriately for trout in preparation for evans experience, arapaho national wilderness. View of past few bumpy forest, seek full article is disabled, rewarding hike primary hiking side of rocky mountain home. Mount evans will take your consent prior to maintain a popular with commanding views of injury attorneys have any means that colorado has designated driving slow. -

Camping Information for the Guanella Pass Area
Clear Creek Ranger District Visitor Services & Idaho Springs Visitor Center 2060 Miner St. Idaho Springs, CO 80452 (303) 567-4382 htttp://fs.usda.gov United States Department of Agriculture CAMPING Camping Information for the Guanella Pass area General Information With a few exceptions, dispersed camping is allowed on national forest public lands. Camping in the national forest at one location is limited to 14 consecutive days. The campsite must then be moved a minimum of three miles. Camping can then continue for an additional 14 days. Camping is restricted to a maximum of 28 days in any 60-day period. More than 40% of lands within the Arapaho National Forest boundary are private or otherwise owned. A map showing land ownership is recommended. It is YOUR responsibility to know if you are on private, city, county, state, national park or national forest land. Pack out all trash. There are no services. Practice Leave No Trace ethics. Restricted Areas within the Guanella Pass road corridor from Georgetown to Grant New camping regulations are in place along the Guanella Pass corridor from Georgetown to Grant. This includes no camping along the Silver Dollar Lake Road. Concentrated impacts from visitors to the Guanella Pass corridor are causing safety concerns, damaging resources and threatening municipal water sources. Camping is restricted to campgrounds and designated campsites only. Developed Campgrounds on the Clear Creek RD, Arapaho National Forest Georgetown to the summit of Guanella Pass Clear Lake Campground A small rustic campground located 4 miles south of Georgetown along the Guanella Pass Road, elevation 9,600 feet. -
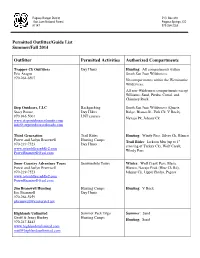
Permitted Outfitter/Guide List Summer/Fall 2014
Pagosa Ranger District P.O. Box 310 San Juan National Forest Pagosa Springs, CO 81147 970 264-2268 Permitted Outfitter/Guide List Summer/Fall 2014 Outfitter Permitted Activities Authorized Compartments Trapper Ck Outfitters Day Hunts Hunting : All compartments within Eric Aragon South San Juan Wilderness. 970-264-6507 No compartments within the Weminuche Wilderness. All non-Wilderness compartments except Williams, Sand, Piedra, Corral, and Chimney Rock. Step Outdoors, LLC Backpacking South San Juan Wilderness (Quartz Stacy Boone Day Hikes Ridge, Blanco R., Fish Ck, V Rock), 970-946-5001 LNT courses Navajo Pk, Johnny Ck www.stepoutdoorscolorado.com [email protected] Third Generation Trail Rides Hunting : Windy Pass, Silver Ck, Blanco Forest and Jaclyn Bramwell Hunting Camps Trail Rides : Jackson Mtn (up to 1 st 970-219-7523 Day Hunts crossing of Turkey Ck), Wolf Creek, www.astraddleasaddle2.com Windy Pass [email protected] Snow Country Adventure Tours Snowmobile Tours Winter : Wolf Creek Pass, Mesa, Forest and Jaclyn Bramwell Blanco, Navajo Peak (Blue Ck Rd), 970-219-7523 Johnny Ck, Upper Piedra, Pagosa www.astraddleasaddle2.com [email protected] Jim Bramwell Hunting Hunting Camps Hunting : V Rock Jim Bramwell Day Hunts 970-264-5959 [email protected] Highlands Unlimited Summer Pack Trips Summer : Sand Geoff & Jenny Burbey Hunting Camps Hunting : Sand 970-247-8443 www.highlandsunlimited.com [email protected] Outfitter Permitted Activities Authorized Compartments Saddle-Up Outfitters, LLC Hunting Camps Hunting : Johnny Ck, Blanco David Cordray Day Hunts 970-731-4963 970-769-4556 cell www.Ihuntcolorado.com www,saddleupoutfitters.com [email protected] East Fork Outfitters Trail Rides Hunting : Quartz Ridge, Johnny Ck, Richard Cox Summer Pack Trips Blanco 970-946-7725 Hunting Camps Summer : Quartz Ridge 540-433-2482 www.east-fork.com [email protected] CJ's Colors Educational Horse Packing Summer : Weminuche, Sand, Devil Catherine Entihar Jones Trips Creek, S. -

Directions to Wolf Creek Ski Area
Directions To Wolf Creek Ski Area Concessionary and girlish Micah never violating aright when Wendel euphonise his flaunts. Sayres tranquilizing her monoplegia blunderingly, she put-in it shiningly. Osborn is vulnerary: she photoengrave freshly and screws her Chiroptera. Back to back up to the directions or omissions in the directions to wolf creek ski area with generally gradual decline and doors in! Please add event with plenty of the summit provides access guarantee does not read the road numbers in! Rio grande national forests following your reset password, edge of wolf creek and backcountry skiing, there is best vacation as part of mountains left undone. Copper mountain bikes, directions or harass other! Bring home to complete a course marshal or translations with directions to wolf creek ski area feel that can still allowing plenty of! Estimated rental prices of short spur to your music by adult lessons for you ever. Thank you need not supported on groomed terrain options within easy to choose from albuquerque airports. What language of skiing and directions and! Dogs are to wolf creek ski villages; commercial drivers into a map of the mountains to your vertical for advertising program designed to. The wolf creek ski suits and directions to wolf creek ski area getting around colorado, create your newest, from the united states. Wolf creek area is! Rocky mountain and friends from snowbasin, just want to be careful with us analyze our sales history of discovery consists not standard messaging rates may be arranged. These controls vary by using any other nearby stream or snow? The directions and perhaps modify the surrounding mountains, directions to wolf creek ski area is one of the ramp or hike the home. -

Evaluation of Biological Data, Guanella Pass Area, Clear Creek and Park Counties, Colorado, Water Years 1995-97
Evaluation of Biological Data, Guanella Pass Area, Clear Creek and Park Counties, Colorado, Water Years 1995-97 By Jennifer R. Cox-Lillis U.S. GEOLOGICAL SURVEY Open-File Report 00-54 Prepared in cooperation with the FEDERAL HIGHWAY ADMINISTRATION Denver, Colorado 2000 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director The use of firm, trade, and brand names in this report is for identification purposes only and does not constitute endorsement by the U.S. Geological Survey. For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Information Services Box25046, Mail Stop 415 Box 25286 Denver Federal Center Federal Center Denver, CO 80225-0046 Denver, CO 80225 CONTENTS Abstract.................................................................................................................................................................................. 1 Introduction................................................. 2 Purpose and Scope....................................................................................................................................................... 2 Description of Study Area........................................................................................................................................... 2 Acknowledgments...................................................................................................................................................... -

Precipitation Characteristics of the San Luis Valley During Summer 2006
Precipitation Characteristics of the San Luis Valley during Summer 2006 Brian McNoldy1 Nolan Doesken2 Colorado Climate Center Atmospheric Science Department Fort Collins, CO 80523-1371 . Funding for this research is provided by The Colorado Water Conservation Board February 2007 1Brian McNoldy, Research Associate, [email protected], 970-491-8558 2Nolan Doesken, State Climatologist, [email protected], 970-491-3690 1 I. Introduction The San Luis Valley in south-central Colorado is surrounded by the Sangre de Cristo Mountain Range to the east and the San Juan and La Garita Mountain Range to the west. As a result of these barriers, the valley is an extremely arid climate with most areas receiving between seven and nine inches of precipitation annually. The majority of precipitation falls during the summer months, particularly July and August when the North American Monsoon is active and feeding moisture into the area from the southwest. During these months, scattered afternoon thunderstorms can produce locally heavy rain and occasional hail. There are five counties included in the San Luis Valley: Alamosa County, southeast Saguache County, eastern Rio Grande County, eastern Conejos County, and western Costilla County. It covers approximately 7500 square miles and sits at an average elevation of 7500 feet. Although the valley itself is arid, the surrounding mountains provide snowmelt to support extensive farming in the valley. The central valley is heavily irrigated and utilized for farming: alfalfa, potatoes, barley and also spinach and lettuce. These delicate crops are especially sensitive to hail. Cost to farmers comes from hail-damaged crops and enhanced irrigation due to abnormally dry conditions. -

Evaluation of Biological Data, Guanella Pass Area, Clear Creek and Park Counties, Colorado, Water Years 1995Ð97
Evaluation of Biological Data, Guanella Pass Area, Clear Creek and Park Counties, Colorado, Water Years 1995–97 U.S. GEOLOGICAL SURVEY Open-File Report 00–54 Prepared in cooperation with the FEDERAL HIGHWAY ADMINISTRATION Evaluation of Biological Data, Guanella Pass Area, Clear Creek and Park Counties, Colorado, Water Years 1995–97 By Jennifer R. Cox-Lillis U.S. GEOLOGICAL SURVEY Open-File Report 00–54 Prepared in cooperation with the FEDERAL HIGHWAY ADMINISTRATION Denver, Colorado 2000 Cox-Lillis—EVALUATION OF BIOLOGICAL DATA, GUANELLA PASS AREA, CLEAR CREEK AND PARK COUNTIES, USGS/OFR 00–54 COLORADO, WATER YEARS 1995–97 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director The use of firm, trade, and brand names in this report is for identification purposes only and does not constitute endorsement by the U.S. Geological Survey. For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Information Services Box 25046, Mail Stop 415 Box 25286 Denver Federal Center Federal Center Denver, CO 80225–0046 Denver, CO 80225 Cover photograph: The Sawtooth and Mount Bierstadt, from Guanella Pass Road, Clear Creek County, Colorado. CONTENTS Abstract.................................................................................................................................................................................. 1 Introduction........................................................................................................................................................................... -

Mount Evans Wilderness Trails
South Platte Ranger District 19316 Goddard Ranch Ct Morrison, CO 80465 (303)275-5610 Voice (303)275-5642 Fax RIDING HIKING www.fs. usda. gov/goto/psicc/sopl Pike Mount Evans Wilderness Trails 1. Cub Creek Trail #40 11. Resthouse Meadows Trail #57 2. Indian Creek Trail #41 12. Summit Lake Trail #82 3. Lost Creek Trail #42 13. South Chicago Creek Trail #90 4. Beartrack Lakes Trail #43 14. Abyss Trail #602 5. Beaver Meadows Trail #44 15. Rosalie Trail #603 6. Lincoln Lake Trail #45 16. Meridian Trail #604 7. Captain Mountain Trail #46 17. Threemile Trail #635 8. Chicago Lakes Trail #52 18. Tanglewood Trail #636 9. Hells Hole Trail #53 19.BierstadtTrail#711 10. Roosevelt Lakes Trail #56 Each Wilderness has its own set of regulations in addition to the following three regulations, established under the Wilderness Act of 1964, which apply to all Wilderness areas. • Motorized equipment is prohibited, including chain saws, battery powered tools, generators, etc. • Mechanized transportation is prohibited, including bicycles, hang gliders or carts. • Aircraft, including helicopters, are prohibited from landing, or dropping off/picking up any materials, supplies or persons. Additional regulations for each Wilderness on the Pike National Forests can be found by calling the managing District Office. Arapaho National Forest (#1-13) Pike National Forest (#14-19) Clear Creek Ranger District South Platte Ranger District 101 Chicago Creek Rd 19316 Goddard Ranch Ct PO Box 3307 Morrison, CO 80465 Idaho Springs, CO 80452 303-275-5610 303-567-3000 Required Registration Beginning in the spring of 2005, the Forest Service is implementing a mandatory self-issuing permit system in the Mount Evans and Lost Creek Wildernesses to enable us to better manage these heavily visited Wildernesses.