ACR-SPR Practice Parameter for Imaging Pregnant Or Potentially
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effects of Alcohol in Newborns Efeitos Do Álcool No Recém-Nascido
REVIEW The effects of alcohol in newborns Efeitos do álcool no recém-nascido Maria dos Anjos Mesquita* ABSTRACT alcoólicas leva a prejuízos individuais, para a sua família e para The purpose of this article was to present a review of the effects toda a sociedade. Apesar disso, a dificuldade do seu diagnóstico e of alcohol consumption by pregnant mothers on their newborn. a inexperiência dos profissionais de saúde faz com que o espectro Definitions, prevalence, pathophysiology, clinical features, diagnostic dessas lesões seja pouco lembrado e até desconhecido. As lesões criteria, follow-up, treatment and prevention were discussed. A causadas pela ação do álcool no concepto são totalmente prevenidas search was performed in Medline, LILACS, and SciELO databases se a gestante não consumir bebidas alcoólicas durante a gestação. using the following terms: “fetus”, “newborn”, “pregnant woman”, Assim, é fundamental a detecção das mulheres consumidoras “alcohol”, “alcoholism”, “fetal alcohol syndrome”, and “alcohol- de álcool durante a gravidez e o desenvolvimento de programas related disorders”. Portuguese and English articles published from específicos de alerta sobre as consequências do álcool durante a 2000 to 2009 were reviewed. The effects of alcohol consumed by gestação e amamentação. pregnant women on newborns are extremely serious and occur frequently; it is a major issue in Public Health worldwide. Fetal alcohol Descritores: Bebidas alcoólicas/efeitos adversos; Feto; Recém-nascido; spectrum disorders cause harm to individuals, their families, and the Síndrome alcoólica fetal; Transtornos relacionados ao uso de álcool entire society. Nevertheless, diagnostic difficulties and inexperience of healthcare professionals result in such damage, being remembered rarely or even remaining uncovered. -

Original Article the Imaging Characteristics of Magnetic Resonance Hysterosalpingography in Infertile Women
Int J Clin Exp Med 2020;13(6):3955-3962 www.ijcem.com /ISSN:1940-5901/IJCEM0107779 Original Article The imaging characteristics of magnetic resonance hysterosalpingography in infertile women Jiugen Ruan1, Chunyan Wu2, Changhua Zhu1, Yao Ding1, Qiuping Tan1, Tao Meng3 Departments of 1Radiology, 2Gynaecology, The People’s Hospital of Xinyu City, Xinyu, Jiangxi, China; 3RIMAG Medical Imaging Corporation, Shanghai, China Received January 13, 2020; Accepted April 1, 2020; Epub June 15, 2020; Published June 30, 2020 Abstract: Objective: We aimed to analyze the imaging characteristics of magnetic resonance hysterosalpingography (MR-HSG) in infertile women. Methods: A total of 20 infertile women admitted to our hospital from October 2018 to December 2019 were selected as the subjects of study for retrospective analysis. The MR-HSG examination was performed in all patients to analyze the examination results and imaging characteristics. Results: (1) The comple- tion rate of MR-HSG examination was 100.00% in the 20 patients, of which those with primary infertility accounted for 65.00% and those with secondary infertility accounted for 35.00%. (2) Among the 20 infertile patients, 30.00% had unobstructed fallopian tubes, 40.00% had partial fallopian tube obstruction, 20.00% had full fallopian tube obstruction and 10.00% had hydrosalpinx. (3) Among the 14 patients with abnormal fallopian tubes, 14.29% had bilateral fallopian tube obstruction, 35.71% had partial fallopian tube obstruction on both sides, 7.14% had fallo- pian tubes that were unobstructed on one side and obstructed on the other side, 21.43% had fallopian tubes that were unobstructed on one side and partially obstructed on the other side, 7.14% had fallopian tubes partially ob- structed on one side and obstructed on the other side, and 14.29% had hydrosalpinx. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -

Hysterosalpingography
H y s t e r o s alp i n g o g r aph y A b o u t P r e p a r a t i o n s Hysterosalpingography is an x-ray examination of the The hysterosalpingography procedure is best uterus and fallopian tubes that uses a special form of x- performed one week after menstruation but before ray called fluoroscopy and a contrast material. During a ovulation to make certain that you are not pregnant hysterosalpingogram, the uterus and fallopian tubes are during the exam. This procedure should not be filled with a contrast material and the radiologist is able to performed if you have an active inflammatory condition. use fluoroscopy to view and assess their anatomy and You should notify your radiologist if you have a chronic function. pelvic infection or an untreated sexually transmitted disease at the time of the procedure. Hysterosalpingography is primarily used to examine women who have difficulty becoming pregnant by On the night before the procedure, you may be asked allowing the radiologist to evaluate the shape and to take a laxative or an enema to empty your bowels, so structure of the uterus, the openness of the fallopian the uterus and surrounding structures can be seen tubes, and any scarring within the uterine or abdominal clearly. Prior to the procedure, you may be given a mild cavity. sedative or over-the-counter medication to minimize any potential discomfort. The exam is used to investigate repeated miscarriages that result from abnormalities in the uterus and to You should inform your radiologist of any medications determine the presence and severity of the following you are taking and if you have any allergies, especially abnormalities: to barium or iodinated contrast materials. -
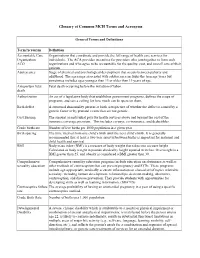
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

If You Are Pregnant: INFORMATION on FETAL DEVELOPMENT, ABORTION and ALTERNATIVES August 2019
If You Are Pregnant: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES August 2019 IF YOU ARE PREGNANT: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES If You Are Pregnant: Information on Fetal Development, Abortion and Alternatives Resources used by the Minnesota Department of Health for this publication are Human Embryology and Developmental Biology, Fifth Edition, 2014; Larsen’s Human Embryology, Fifth Edition, 2014; The Developing Human, 10th Edition, 2016; and In the Womb, 2006. The photographs in this booklet are credited to Lennart Nilsson/TT Images and are used by permission; except for week 38 copyright Minnesota Department of Health. The illustrations found throughout this booklet were created by Peg Gerrity, Houston, Texas. Copyright: http://www.peggerrity.com. Minnesota Department of Health Division of Child and Family Health PO Box 64882 St. Paul, MN 55164-0882 651-201-3580 Women's Right to Know (https://www.health.state.mn.us/people/wrtk/index.html) Upon request, this material will be made available in an alternative format such as large print, Braille or audio recording. Printed on recycled paper. 2 IF YOU ARE PREGNANT: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES Contents If You Are Pregnant: INFORMATION ON FETAL DEVELOPMENT, ABORTION AND ALTERNATIVES............................................................................................................................. 1 Introduction ............................................................................................................................... -

A B C Pregnancy Terms and Definitions
Pregnancy Terms and Definitions Obstetrics & Gynecology A After pains or afterbirth pains: Contractions of the uterus that occur after your baby is born, as the uterus returns to its normal size. This may cause cramping for a few days, especially if this is not your first baby or if you are nursing. Amniocentesis: the removal of a sample of amniotic fluid by means of a needle inserted through the mother’s abdominal wall; used for genetic and biochemical analysis of the baby. Amniotic fluid: the liquid surrounding and protecting the baby within the amniotic sac throughout pregnancy. Amniotic sac: the membrane within the uterus that contains the baby and the amniotic fluid. Analgesic: Medication that relieves or reduces pain. Anesthesia: Loss of feeling. There are three ways of doing this: general, local and epidural. Anesthesiologist: A doctor who specializes in the use of anesthesia. Anesthetist: A registered nurse who has special training in anesthesia. Apgar score rating: A system to evaluate the health of your baby immediately after birth. The score can be zero to 10, based on appearance and color, pulse, reflexes, activity and respiration. B Baby blues: A mild depression many women feel in the first few weeks after birth. Braxton-Hicks contractions: Mild, usually painless contractions that occur during the entire pregnancy, but are only felt from the 5th month on. Breech birth: Baby is born feet or buttocks first. C Cephalopelvic disproprition (CPD): Baby’s head is too large for the mother’s pelvic bones. Cervix: the neck of the uterus; Pap smears are taken from the cervix. -

Procedure Codes for Physician: Radiology
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN - PROCEDURE CODES SECTION 4 - RADIOLOGY Physician – Procedure Codes, Section 4 - Radiology Table of Contents GENERAL INSTRUCTIONS ............................................................................................................ 4 GENERAL RULES AND INFORMATION ......................................................................................... 6 MMIS RADIOLOGY MODIFIERS .................................................................................................... 8 DIAGNOSTIC RADIOLOGY (DIAGNOSTIC IMAGING)................................................................. 9 HEAD AND NECK.................................................................................................................... 9 CHEST .................................................................................................................................. 10 SPINE AND PELVIS .............................................................................................................. 11 UPPER EXTREMITIES .......................................................................................................... 12 LOWER EXTREMITIES ......................................................................................................... 13 ABDOMEN ............................................................................................................................ 14 GASTROINTESTINAL TRACT ............................................................................................... 15 URINARY -

Alcohol Abuse in Pregnant Women: Effects on the Fetus and Newborn, Mode of Action and Maternal Treatment
Int. J. Environ. Res. Public Health 2010, 7, 364-379; doi:10.3390/ijerph7020364 OPEN ACCESS International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph Review Alcohol Abuse in Pregnant Women: Effects on the Fetus and Newborn, Mode of Action and Maternal Treatment Asher Ornoy 1,* and Zivanit Ergaz 1,2 1 Laboratory of Teratology, The Institute of Medical Research Israel Canada, Hadassah Medical School and Hospital, The Hebrew University of Jerusalem, Ein Kerem, P.O. Box 12271, Jerusalem, 91120, Israel; E-Mail: [email protected] 2 Department of Neonatology, Hadassah Medical School and Hospital, Hadassah Medical Center, Hebrew University, P.O. Box 24035, Jerusalem, 91240, Israel * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +972-50-624-2125. Received: 16 December 2009 / Accepted: 22 January 2010 / Published: 27 January 2010 Abstract: Offspring of mothers using ethanol during pregnancy are known to suffer from developmental delays and/or a variety of behavioral changes. Ethanol, may affect the developing fetus in a dose dependent manner. With very high repetitive doses there is a 6–10% chance of the fetus developing the fetal alcoholic syndrome manifested by prenatal and postnatal growth deficiency, specific craniofacial dysmorphic features, mental retardation, behavioral changes and a variety of major anomalies. With lower repetitive doses there is a risk of "alcoholic effects" mainly manifested by slight intellectual impairment, growth disturbances and behavioral changes. Binge drinking may impose some danger of slight intellectual deficiency. It is advised to offer maternal abstinence programs prior to pregnancy, but they may also be initiated during pregnancy with accompanying close medical care. -

Prenatal Development
2 Prenatal Development Learning Objectives Conception and Genetics 2.5 What behaviors have scientists observed 2.8 How do maternal diseases and 2.1 What are the characteristics of the zygote? in fetuses? environmental hazards affect prenatal 2.1a What are the risks development? associated with assisted Problems in Prenatal Development 2.8a How has technology changed reproductive technology? 2.6 What are the effects of the major dominant, the way that health professionals 2.2 In what ways do genes influence recessive, and sex-linked diseases? manage high-risk pregnancies? development? 2.6a What techniques are used to as- 2.9 What are the potential adverse effects sess and treat problems in prena- of tobacco, alcohol, and other drugs on Development from Conception to Birth tal development? prenatal development? 2.3 What happens in each of the stages of 2.7 How do trisomies and other disorders of 2.10 What are the risks associated with legal prenatal development? the autosomes and sex chromosomes drugs, maternal diet, age, emotional 2.4 How do male and female fetuses differ? affect development? distress, and poverty? efore the advent of modern medical technology, cul- garments that are given to her by her mother. A relative ties tures devised spiritual practices that were intended to a yellow thread around the pregnant woman’s wrist as cer- B ensure a healthy pregnancy with a happy outcome. emony attendees pronounce blessings on the unborn child. For instance, godh bharan is a centuries-old Hindu cere- The purpose of the thread is to provide mother and baby mony that honors a woman’s first pregnancy. -

FETAL GROWTH and DEVELOPMENT Copyright© 1995 by the South Dakota Department of Health
FETAL GROWTH AND DEVELOPMENT Copyright© 1995 by the South Dakota Department of Health. All rights reserved. The South Dakota Department of Health acknowledges Keith L. Moore, Ph.D., F.I.A.C, F.R.S.M.; T.V.N. Persaud, M.D., Ph.D., F.R.C. Path (Lond): Cynthia Barrett, M.D.; and Kathleen A. Veness-Meehan, M.D.; for their professional assis- tance in reviewing this booklet. Photos on pages 8, 9, 11, 13, 16 and 18 by Lennart Nilsson, of Sweden, A Child is Born, 1986, Dell Publishing and are used by permission. Lennart Nilsson is a pioneer in medical photography, credited with inventing numerous devices and techniques in his field. The photos used in this booklet have been published internationally in scientific periodicals and used in the popular press and television. Illustrations on pp. 5 and 6 by Drs. K.L. Moore, T.V.N. Persaud and K. Shiota, Color Atlas of Clinical Embryology, 1944, Philadelphia: W.B. Saunders, are used by permission. The South Dakota Department of Health also acknowledges the technical assis- tance of the following in development of the booklet: Gary Crum, Ph.D., Ann Kappel and Arlen Pennell of the Ohio Department of Health; Sandra Van Gerpen, M.D. M.P.H., Terry Englemann, R.N., Colleen Winter, R.N., B.S.N., and Nancy Shoup, R.N., B.S.N., of the South Dakota Department of Health; Dennis Stevens, M.D.; Virginia Johnson, M.D.; Brent Lindbloom, D.O.; Dean Madison, M.D.; Buck Williams, M.D.; Roger Martin, R.N., C.N.P., M.S.; Barbara Goddard, B.S., Ph.D.; Laurie Lippert; Vincent Rue, P.h.D.; and Representative Roger Hunt. -

Zwanger-Pesiri Radiology Provides the Latest Ability to Detect, Characterize, Stage Ultrasound (Sonography) Equipment at Each of and Treat Disease Our Offices
ESIRI P At the Forefront of Forefront the At WANGER- Z Technology, Diagnosis & Care Technology, 521 Route 111 Stony Brook Medical Park Hauppauge 11788 2500 Nesconset Hwy, Bldg 15 Stony Brook 11790 80 Maple Avenue 25A Smithtown 11787 220 Belle Mead Rd 763 Larkfield Road R Commack 11725 347 East Setauket 11733 - E 25A W - 680 Old Country Rd i 25A l l i 2087 Deer Park Ave a Plainview 11803 25 m Deer Park 11729 N G i F c 112 l 1500 William Floyd Pkwy - 111 o o y l l d s Shirley 11967 272 North Broadway kwy P P 25 R 495 k N Hicksville 11801 the t e d w r rn - No Sta 231 y S 495 a g 111 454 27 A t i 495 k o 1390 Hempstead Tpke Suffolk Ave - s Brookhaven Prof. Park 11003 Wantagh Pkwy P Elmont y M k w 135 106 w k ead North Ocean Plaza 285 Sills Road, Bldg 15 o kwy y P Hempstead Tpke P W w 107 te ta b S 1729 N. Ocean Ave Patchogue 11772 d n - r r n e o a South 443 Sunrise Hwy l 24 o 27 Medford 11763 s k I Z zprad.com Lynbrook 11563 s P s k 27A o r w lt Pkwy C y Be S nrise u Hwy 759 Montauk Hwy 160 Brentwood Rd 2012 Sunrise Hwy West Islip 11795 Bay Shore 11706 516 631 Merrick 11566 126 Hicksville Rd Massapequa 11758 150 E. Sunrise Hwy Lindenhurst 11757 CVS Plaza 355 Broadway Amityville 11701 C ontinuum of C are Echocardiography Two-dimensional imaging of the cardiovascular system Confidence Accurate assessment of the velocity Unsurpassed diagnostic technology of blood, cardiac tissue and cardiac valves The most experience with 3.0 Tesla MRI and 3D Mammography on Long Island Visualizes leaking of blood through the valves (valvular regurgitation)