RAD5A, RECQ4A, and MUS81 Have Specific Functions in Homologous
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Topoisomerases and Cancer Topoisomerases and TOP Genes in Humans Humans Vs
DNA Topoisomerases Review DNA Topoisomerases And Cancer Topoisomerases and TOP Genes in Humans Humans vs. Escherichia Coli Topoisomerase differences Comparisons Topoisomerase and genomes Top 1 Top1 and Top2 differences Relaxation of DNA Top1 DNA supercoiling DNA supercoiling In the context of chromatin, where the rotation of DNA is constrained, DNA supercoiling (over- and under-twisting and writhe) is readily generated. TOP1 and TOP1mt remove supercoiling by DNA untwisting, acting as “swivelases”, whereas TOP2a and TOP2b remove writhe, acting as “writhases” at DNA crossovers (see TOP2 section). Here are some basic facts concerning DNA supercoiling that are relevant to topoisomerase activity: • Positive supercoiling (Sc+) tightens the DNA helix whereas negative supercoiling (Sc-) facilitates the opening of the duplex and the generation of single-stranded segments. • Nucleosome formation and disassembly absorbs and releases Sc-, respectively. • Polymerases generate Sc+ ahead and Sc- behind their tracks. • Excess of Sc+ arrests DNA tracking enzymes (helicases and polymerases), suppresses transcription elongation and initiation, and destabilizes nucleosomes. • Sc- facilitates DNA melting during the initiation of replication and transcription, D-loop formation and homologous recombination and nucleosome formation. • Excess of Sc- favors the formation of alternative DNA structures (R-loops, guanine quadruplexes, right-handed DNA (Z-DNA), plectonemic structures), which then absorb Sc- upon their formation and attract regulatory proteins. The -

Type IA Topoisomerases As Targets for Infectious Disease Treatments
microorganisms Review Type IA Topoisomerases as Targets for Infectious Disease Treatments Ahmed Seddek 1,2 , Thirunavukkarasu Annamalai 1 and Yuk-Ching Tse-Dinh 1,2,* 1 Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA; asedd001@fiu.edu (A.S.); athiruna@fiu.edu (T.A.) 2 Biomolecular Sciences Institute, Florida International University, Miami, FL 33199, USA * Correspondence: ytsedinh@fiu.edu; Tel.: +1-305-348-4956 Abstract: Infectious diseases are one of the main causes of death all over the world, with antimi- crobial resistance presenting a great challenge. New antibiotics need to be developed to provide therapeutic treatment options, requiring novel drug targets to be identified and pursued. DNA topoi- somerases control the topology of DNA via DNA cleavage–rejoining coupled to DNA strand passage. The change in DNA topological features must be controlled in vital processes including DNA repli- cation, transcription, and DNA repair. Type IIA topoisomerases are well established targets for antibiotics. In this review, type IA topoisomerases in bacteria are discussed as potential targets for new antibiotics. In certain bacterial pathogens, topoisomerase I is the only type IA topoisomerase present, which makes it a valuable antibiotic target. This review will summarize recent attempts that have been made to identify inhibitors of bacterial topoisomerase I as potential leads for antibiotics and use of these inhibitors as molecular probes in cellular studies. Crystal structures of inhibitor–enzyme complexes and more in-depth knowledge of their mechanisms of actions will help to establish the structure–activity relationship of potential drug leads and develop potent and selective therapeutics that can aid in combating the drug resistant bacterial infections that threaten public health. -

SMX Makes the Cut in Genome Stability
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 61), pp: 102765-102766 Editorial SMX makes the cut in genome stability Haley D.M. Wyatt and Stephen C. West The faithful duplication and preservation of our SLX4 scaffold co-ordinate three different nucleases for genetic material is essential for cell survival; however, DNA cleavage? SMX was found to be a promiscuous DNA is susceptible to damage by extracellular and endonuclease that cleaves a broad range of DNA secondary intracellular agents (e.g. ultraviolet radiation, reactive structures in vitro3. Remarkably, SLX4 co-ordinates the oxygen species). DNA double-strand breaks (DSBs) SLX1 and MUS81-EME1 nucleases during HJ resolution are thought to represent the most dangerous type of [3, 4] (Figure 1). The involvement of two active sites from lesion, as the failure to repair a DSB can lead to loss of different heterodimeric enzymes leads to a non-canonical genetic information, chromosomal rearrangements, and mechanism of HJ resolution. It was also shown that SLX4 cell death. Fortunately, cells contain sophisticated DNA activates MUS81-EME1 to cleave structures that resemble repair pathways to counteract the deleterious effects of stalled replication forks [3] (Figure 1). Activation involves genotoxic agents. Mutations in DNA repair genes are relaxation of MUS81-EME1’s substrate specificity, which linked to various diseases, including neurological defects, is regulated by a helix-hairpin-helix (HhH) domain in immunodeficiency, and cancer. the MUS81 N-terminus (MUS81 N-HhH). Intriguingly, Gross chromosomal rearrangements, including MUS81 N-HhH also mediates the interaction with deletions, duplications, and translocations, are a hallmark SLX4 via a C-terminal SAP domain (SLX4 SAP) [5]. -

The Choice in Meiosis – Defining the Factors That Influence Crossover Or Non-Crossover Formation
Commentary 501 The choice in meiosis – defining the factors that influence crossover or non-crossover formation Jillian L. Youds and Simon J. Boulton* DNA Damage Response Laboratory, Cancer Research UK, London Research Institute, Clare Hall, Blanche Lane, South Mimms EN6 3LD, UK *Author for correspondence ([email protected]) Journal of Cell Science 124, 501-513 © 2011. Published by The Company of Biologists Ltd doi:10.1242/jcs.074427 Summary Meiotic crossovers are essential for ensuring correct chromosome segregation as well as for creating new combinations of alleles for natural selection to take place. During meiosis, excess meiotic double-strand breaks (DSBs) are generated; a subset of these breaks are repaired to form crossovers, whereas the remainder are repaired as non-crossovers. What determines where meiotic DSBs are created and whether a crossover or non-crossover will be formed at any particular DSB remains largely unclear. Nevertheless, several recent papers have revealed important insights into the factors that control the decision between crossover and non-crossover formation in meiosis, including DNA elements that determine the positioning of meiotic DSBs, and the generation and processing of recombination intermediates. In this review, we focus on the factors that influence DSB positioning, the proteins required for the formation of recombination intermediates and how the processing of these structures generates either a crossover or non-crossover in various organisms. A discussion of crossover interference, assurance and homeostasis, which influence crossing over on a chromosome-wide and genome-wide scale – in addition to current models for the generation of interference – is also included. This Commentary aims to highlight recent advances in our understanding of the factors that promote or prevent meiotic crossing over. -

Cleavage Mechanism of Human Mus81–Eme1 Acting on Holliday-Junction Structures
Cleavage mechanism of human Mus81–Eme1 acting on Holliday-junction structures Ewan R. Taylor* and Clare H. McGowan*†‡ Departments of *Molecular Biology and †Cell Biology, The Scripps Research Institute, La Jolla, CA 92037 Edited by Stephen J. Elledge, Harvard Medical School, Boston, MA, and approved January 10, 2008 (received for review October 29, 2007) Recombination-mediated repair plays a central role in maintaining Results genomic integrity during DNA replication. The human Mus81–Eme1 Recombinant Human Mus81–Eme1. Truncation analysis of both endonuclease is involved in recombination repair, but the exact Mus81 and Eme1 was used to define the minimal domains structures it acts on in vivo are not known. Using kinetic and enzy- required for endonuclease function [see supplementary infor- matic analysis of highly purified recombinant enzyme, we find mation (SI) Fig. 5]. Versions of each protein containing amino that Mus81–Eme1 catalyzes coordinate bilateral cleavage of acids 260–551 for Mus81 and amino acids 244–571 for Eme1 model Holliday-junction structures. Using a self-limiting, cruciform- were expressed in Escherichia coli. The recombinant truncated containing substrate, we demonstrate that bilateral cleavage occurs complex, which we named EcME, was well expressed, largely sequentially within the lifetime of the enzyme–substrate complex. soluble, and, as detailed below, active. The complex was purified Coordinate bilateral cleavage is promoted by the highly cooperative to apparent homogeneity through affinity chromatography, ion nature of the enzyme and results in symmetrical cleavage of a exchange, and gel filtration steps (Fig. 1a). cruciform structure, thus, Mus81–Eme1 can ensure coordinate, bilat- eral cleavage of Holliday junction-like structures. -

Regulation of Mus81-Eme1 Structure-Specific Endonuclease by Eme1 SUMO-Binding and Rad3atr Kinase Is Essential in the Absence of Rqh1blm Helicase
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.29.454171; this version posted July 29, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Regulation of Mus81-Eme1 structure-specific endonuclease by Eme1 SUMO-binding and Rad3ATR kinase is essential in the absence of Rqh1BLM helicase Giaccherini C.1,#, Scaglione S. 1, Coulon S. 1, Dehé P.M.1,#,* and Gaillard P.H.L. 1* 1 Centre de Recherche en Cancérologie de Marseille, CRCM, Inserm, CNRS, Aix- Marseille Université, Institut Paoli-Calmettes, Marseille, France. #The authors contributed equally *correspondence [email protected] [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.29.454171; this version posted July 29, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract The Mus81-Eme1 structure-specific endonuclease is crucial for the processing of DNA recombination and late replication intermediates. In fission yeast, stimulation of Mus81-Eme1 in response to DNA damage at the G2/M transition relies on Cdc2CDK1 and DNA damage checkpoint-dependent phosphorylation of Eme1 and is critical for chromosome stability in absence of the Rqh1BLM helicase. Here we identify Rad3ATR checkpoint kinase consensus phosphorylation sites and two SUMO interacting motifs (SIM) within a short N-terminal domain of Eme1 that is required for cell survival in absence of Rqh1BLM. -
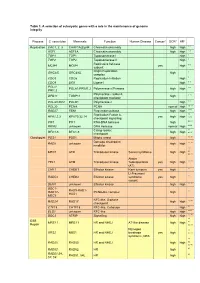
Table 1. a Selection of Eukaryotic Genes with a Role in the Maintenance of Genome Integrity
Table 1. A selection of eukaryotic genes with a role in the maintenance of genome integrity Process S. cerevisiae Mammals Function Human Disease Cancer* GCR* HR* Replication CAC1, 2, 3 CHAF1A,B,p48 Chromatin assembly high high 1, 2 1, 2 ASF1 ASF1A Chromatin assembly high high 3 TOP1 TOP1 Topoisomerase I high 3 TOP2 TOP2 Topoisomerase II high Replicative helicase 4, 5 MCM4 MCM4 yes high subunit Origin Replication 6 ORC3/5 ORC3/6L high complex 7 CDC6 CDC6 Replication Initiation high 7, 8 CDC9 LIG1 Ligase I high POL1/ 7-10 POLA1/PRIM1,2 Polymerase α/Primase high high PRI1,2 Polymerase ε subunit, 6, 11 DPB11 TOBP11 high checkpoint mediator POL3/CDC2 POLD1 Polymerase δ high 7, 8 POL30 PCNA PCNA normal high 12, 13 RAD27 FEN1 Flap endonuclease high high 14-16 Replication Factor A, 14, RFA1,2,3 RPA70,32,14 yes high high 17-19 checkpoint signalling PIF1 PIF1 RNA-DNA helicase high 20, 21 RRM3 unknown DNA Helicase normal high 22-26 Clamp loader, 11, RFC1-5 RFC1-5 high high 20, 27 checkpoint Checkpoint PDS1 PDS1 Mitotic arrest high 11, 28 Damage checkpoint 11, 29 RAD9 unknown high high mediator 11, MEC1 ATR Transducer kinase Seckel syndrome high high 28, 30, 31 Ataxia TEL1 ATM Transducer kinase Telangiectasia yes high high 11, 28 (AT) CHK1 CHEK1 Effector kinase Rare tumours yes high 11 Li-Fraumeni RAD53 CHEK2 Effector kinase syndrome yes high 11 variant DUN1 unknown Effector kinase high high 11, 32 DDC1- RAD9-RAD1- 11 RAD17- PCNA-like complex high HUS1 MEC3 RFC-like, S-phase 11, 33 RAD24 RAD17 high high checkponit 34 CTF18 CHTF18 RFC-like, Cohesion -

Identification of Gastric Cancer–Related Genes Using a Cdna Microarray Containing Novel Expressed Sequence Tags Expressed in Gastric Cancer Cells
Vol. 11, 473–482, January 15, 2005 Clinical Cancer Research 473 Identification of Gastric Cancer–Related Genes Using a cDNA Microarray Containing Novel Expressed Sequence Tags Expressed in Gastric Cancer Cells Jeong-Min Kim,1,5 Ho-Yong Sohn,4 overexpressed in z68% of tissues and the MT2A gene Sun Young Yoon,1 Jung-Hwa Oh,1 Jin Ok Yang,1 was down-expressed in 72% of the tissues. Western blotting and immunohistochemical analyses for CDC20 and SKB1 Joo Heon Kim,2 Kyu Sang Song,3 Seung-Moo Rho,2 1 1 5 showed overexpression and localization changes of the Hyan Sook Yoo, Yong Sung Kim, Jong-Guk Kim, corresponding protein in human gastric cancer tissues. 1 and Nam-Soon Kim Conclusions: Novel genes that are related to human 1Genome Research Center, Korea Research Institute of Bioscience and gastric cancer were identified using cDNA microarray Biotechnology; 2Department of Pathology, Eulji University School of 3 developed in our laboratory. In particular, CDC20 and Medicine; and Department of Pathology, College of Medicine, MT2A represent a potential biomarker of human gastric Chungnam National University, Daejeon, Korea; 4Department of Food and Nutrition, Andong National University, Andong, Korea; and cancer. These newly identified genes should provide a 5Department of Microbiology, College of Natural Sciences, Kyungpook valuable resource for understanding the molecular mecha- National University, Daegu, Korea nism associated with tumorigenesis of gastric carcinogenesis and for the discovery of potential diagnostic markers of gastric cancer. ABSTRACT Purpose: Gastric cancer is one of the most frequently INTRODUCTION diagnosed malignancies in the world, especially in Korea and Japan. -

Two Closely Related Recq Helicases Have Antagonistic Roles in Homologous Recombination and DNA Repair in Arabidopsis Thaliana
Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana Frank Hartung, Stefanie Suer, and Holger Puchta* Botany II, University Karlsruhe, Kaiserstrasse 12, D-76128 Karlsruhe, Germany Edited by Nina Fedoroff, U.S. Department of State, Washington, DC, and approved October 4, 2007 (received for review June 26, 2007) RecQ helicases are involved in the processing of DNA structures RMI1 protein was characterized in mammals and yeast (10, 18, arising during replication, recombination, and repair throughout all 19). Moreover, in the lower eukaryotes S. cerevisiae and Schizo- kingdoms of life. Mutations of different RecQ homologues are re- saccharomyces pombe deletion of their respective RecQ homo- sponsible for severe human diseases, such as Blooms (BLM) or Werner logue leads to partial suppression of the severe phenotypes (WRN) syndrome. The loss of RecQ function is often accompanied by caused by mutation of the TOP3 gene (14, 20–22). hyperrecombination caused by a lack of crossover suppression. In the Several RecQ helicase mutants are synthetically lethal in a Arabidopsis genome seven different RecQ genes are present. Two of combination with mutations in the endonuclease MUS81 (9, 23, them (AtRECQ4A and 4B) arose because of a recent duplication and 24). This inviability of the double mutants is most probably because are still nearly 70% identical on a protein level. Knockout of these both proteins act in parallel pathways resolving aberrant DNA genes leads to antagonistic phenotypes: the RECQ4A mutant shows structures arising during replication. When both genes are mutated, sensitivity to DNA-damaging agents, enhanced homologous recom- these structures accumulate, leading to cell death (9, 23). -

(12) United States Patent (10) Patent No.: US 8,148,129 B2 Frankel Et Al
US008148129B2 (12) United States Patent (10) Patent No.: US 8,148,129 B2 Frankel et al. (45) Date of Patent: Apr. 3, 2012 (54) GENERATION OF POTENT DOMINANT 6,824,978 B1 1 1/2004 Cox, III et al. NEGATIVE TRANSCRIPTIONAL 6,933,113 B2 8, 2005 Case et al. 6,979,539 B2 12/2005 Cox, III et al. INHIBITORS 7,013,219 B2 3/2006 Case et al. 7,070,934 B2 7/2006 Cox, III et al. (75) Inventors: Alan Frankel, Mill Valley, CA (US); 7,163,824 B2 1/2007 Cox, III et al. Robert Nakamura, San Francisco, CA 7,220,719 B2 5/2007 Case et al. (US); Chandreyee Das, Brookline, MA 7,235,354 B2 6/2007 Case et al. 7,262,054 B2 8/2007 Jamieson et al. (US); Ivan D’Orso, San Francisco, CA 7,273,923 B2 9/2007 Jamieson et al. (US); Jocelyn Grunwell, San Mateo, 2003, OO82552 A1* 5, 2003 Wolffe et al. ..................... 435/6 CA (US) (73) Assignee: The Regents of the University of OTHER PUBLICATIONS California, Oakland, CA (US) Cramer et al., Coupling of Transcription with Alternative Splicing: RNA Pol II Promoters Modulate SF2. ASF and 9G8 Effects on an (*) Notice: Subject to any disclaimer, the term of this Exonic Splicing Enhancer, Molecular Cell, 1999, 4:251-258.* patent is extended or adjusted under 35 Cama-Carvalho et al., Nucleocytoplasmic shuttling of heterodimeric U.S.C. 154(b) by 806 days. splicing factor U2AF, JBC. Published on Dec. 15, 2000 as Manu script M008759200.* (21) Appl. No.: 11/765,592 Rosonina et al., Gene Expression: The Close Coupling of Transcrip tion and Splicing, Current Biology, vol. -

Rare Coding Variants in Five DNA Damage Repair Genes Associate
medRxiv preprint doi: https://doi.org/10.1101/2021.04.18.21255506; this version posted April 20, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license . 1 Rare coding variants in five DNA damage repair genes 2 associate with timing of natural menopause 3 Lucas D. Ward1*, Margaret M. Parker1, Aimee M. Deaton1, Ho-Chou Tu1, Alexander O. Flynn-Carroll1, 4 Gregory Hinkle1, Paul Nioi1 5 6 1. Alnylam Pharmaceuticals, Cambridge, MA 02142, USA. 7 * To whom correspondence should be addressed. Email: [email protected]. 8 9 Abstract 10 The age of menopause is associated with fertility and disease risk, and its genetic control is of great 11 interest. We used whole-exome sequences from 119,992 women in the UK Biobank to test for 12 associations between rare damaging variants and age at natural menopause. Rare damaging variants in 13 three genes significantly associated with menopause: CHEK2 (p = 6.2 x 10-51) and DCLRE1A (p = 1.2 x 14 10-12) with later menopause and TOP3A (p = 8.8 x 10-8) with earlier menopause. Two additional genes 15 were suggestive: RAD54L (p = 2.3 x 10-6) with later menopause and HROB (p = 2.7 x 10-6) with earlier 16 menopause. In a follow-up analysis of repeated questionnaires in women who were initially pre- 17 menopausal, CHEK2, TOP3A, and RAD54L genotype associated with subsequent menopause. -

The MUS81 Endonuclease Is Essential for Telomerase Negative Cell Proliferation Sicong Zeng Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 The MUS81 endonuclease is essential for telomerase negative cell proliferation Sicong Zeng Washington University School of Medicine in St. Louis Qin Yang Washington University School of Medicine in St. Louis Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Zeng, Sicong and Yang, Qin, ,"The UM S81 endonuclease is essential for telomerase negative cell proliferation." Cell Cycle.8,15. 2157-2160. (2009). https://digitalcommons.wustl.edu/open_access_pubs/3006 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. [Cell Cycle 8:15, 2157-2160; 1 August 2009]; ©2009 Landes Bioscience Extra View The MUS81 endonuclease is essential for telomerase negative cell proliferation Sicong Zeng† and Qin Yang* Department of Radiation Oncology; Washington University School of Medicine; St. Louis, MO USA †Present address: Institute of Reproduction and Stem Cell Engineering; Central South University; Changsha, Hunan China Key words: ALT, APBs, MUS81, telomere recombination A substantial number of human tumors (~10%) are telomerase breaks and maintains telomere length and stability. Progressive negative, and cells in such tumors have been proposed to main- telomere shortening in normal cells during DNA replication leads tain telomere length by the alternative lengthening of telomeres eventually to a permanent halt of cell division referred to as replica- (ALT) pathway. Although details of the molecular mechanism tive senescence. This end replication problem of human telomeres of ALT are largely unknown, previous studies have shown that has received particular attention due to its implications in ageing telomere homologous recombination (HR) is implicated in the and cancer.