The Roles of Membrane Technology in Artificial Organs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Total Artificial Heart
The First Total Artificial Heart On the night of December 1-2, 1982, with a major winter storm howling outside, medical history was being made inside the University of Utah Hospital. This event was the implantation of the first destination total artificial heart (TAH) in a human being. That person, 61-year-old Barney Clark, was a retired Seattle dentist with family roots in Utah. Dr. Clark’s several year history of dyspnea and fatigability had been attributed to chronic obstructive pulmonary disease in a University of Utah Hospital former smoker. However, 2 1/2 years before admission, a diagnosis of heart failure was made associated with atrial fibrillation with a rapid ventricular response. In-patient treatment for recurrent heart failure with paroxysmal ventricular tachycardia (VT) was required 1 ½ years before admission. Coronary angiography and left ventriculography established a diagnosis of advanced, non-ischemic dilated cardiomyopathy with an ejection fraction of 23%. Because of symptomatic progression of heart failure, Dr. Clark was referred to the author at LDS Hospital in Salt Lake City, near family members, for investigational inotrope therapy (amrinone), but this caused hypotension and exacerbated atrial and ventricular tachyarrhythmias. Endomyocardial biopsy showed low-grade cellular and humoral myocarditis, and a course of immunosuppressant therapy (prednisone and azathioprine) was begun with initial improvement. However, clinical deterioration resumed, with low- output failure and edema, 6 ½ months later, leading to hospitalization for IV diuretics and dobutamine. Clinical improvement was only marginal, leaving him in class IV heart failure. Jeffrey L. Anderson, MD: Barney Clark’s Cardiologist An opportune meeting occurred 3-4 months prior to the final admission between the author and Dr. -

Ventricular Assist Devices (Vads) and Total Artificial Hearts These Services May Or May Not Be Covered by Your Healthpartners Plan
Ventricular assist devices (VADs) and total artificial hearts These services may or may not be covered by your HealthPartners plan. Please see your plan documents for your specific coverage information. If there is a difference between this general information and your plan documents, your plan documents will be used to determine your coverage. Administrative Process Prior authorization is required for insertion of an implantable ventricular assist device (VAD). Prior authorization is required for placement of a total artificial heart (TAH). Prior authorization is not required in the event that either of the devices listed above is used under emergency circumstances for a critically ill member in an in-patient setting. Emergency use is defined as necessary to save the life or protect the immediate well-being of a given patient. However, services with specific coverage criteria may be reviewed concurrently or retrospectively to determine medical necessity. Prior authorization is not required for percutaneous ventricular assist devices (pVADs). Coverage Insertion of an implantable ventricular assist device (VAD) or total artificial heart (TAH) is covered per the member’s plan documents when the criteria outlined below are met and the procedure is performed at a HealthPartners Transplant Center of Excellence. Please see the Related Content section for the Transplant Centers of Excellence documents. Indications that are covered Implantable ventricular assist device Adult 1. An implantable VAD is covered as a bridge to recovery in patients with a potentially reversible condition when the following criteria are met: A. The requested device has received approval from the Food and Drug Administration (FDA) and is being used in accordance with device-specific, FDA-approved indications. -

The Artificial Heart: Costs, Risks, and Benefits
The Artificial Heart: Costs, Risks, and Benefits May 1982 NTIS order #PB82-239971 THE IMPLICATIONS OF COST-EFFECTIVENESS ANALYSIS OF MEDICAL TECHNOLOGY MAY 1982 BACKGROUND PAPER #2: CASE STUDIES OF MEDICAL TECHNOLOGIES CASE STUDY #9: THE ARTIFICIAL HEART: COST, RISKS, AND BENEFITS Deborah P. Lubeck, Ph. D. and John P. Bunker, M.D. Division of Health Services Research, Stanford University Stanford, Calif. Contributors: Dennis Davidson, M. D.; Eugene Dong, M. D.; Michael Eliastam, M. D.; Dennis Florig, M. A.; Seth Foldy, M. D.; Margaret Marnell, R. N., M. A.; Nancy Pfund, M. A.; Thomas Preston, M. D.; and Alice Whittemore, Ph.D. OTA Background Papers are documents containing information that supplements formal OTA assessments or is an outcome of internal exploratory planning and evalua- tion. The material is usually not of immediate policy interest such as is contained in an OTA Report or Technical Memorandum, nor does it present options for Con- gress to consider. -I \lt. r,,, ,.~’ . - > ‘w, . ,+”b Office of Technology Assessment ./, -. Washington, D C 20510 4,, P ---Y J, ,,, ,,,, ,,, ,. Library of Congress Catalog Card Number 80-600161 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 Foreword This case study is one of 17 studies comprising Background Paper #2 for OTA’S assessment, The Implications of Cost-Effectiveness Analysis of Medical Technology. That assessment analyzes the feasibility, implications, and value of using cost-effec- tiveness and cost-benefit analysis (CEA/CBA) in health -

8 Implantable Membrane Oxygenator
Artificial Lung 8 . Cardiopulmonary Bypass - oxgenator 1 Cardiopulmonary Bypass § Cardiopulmonary bypass (CPB), also called heart-lung bypass, allows the temporary replacement of the gas exchange function of the lungs and the blood-pumping function of the heart § The terms pump-oxygenator and heart–lung machine graphically describe the equipment used § By extension, the term is also applies to surgical procedures which take place primarily on the external aspects of the heart, such as creating new routes for blood to reach the distal coronary arteries from the aorta 3 Cardiopulmonary Bypass § As usually employed for cardiac surgery, the heart–lung machine is part of a total, venoarterial cardiopulmonary bypass circuit à meaning that all the venous blood returning to the right heart cavities is collected in the extracorporeal circuit à circulated through the gas exchange device, from where it is pumped back into the arterial tree à thereby “bypassing” the heart cavities and the pulmonary circulation 5 Cardiopulmonary Bypass Scheme of standard operating conditions during cardiopulmonary bypass 6 Cardiopulmonary Bypass 7 Artificial Lung Artificial Lung vs. Natural Lung 8 Artificial Lung vs. Natural Lung § In the natural lungs, the factors underlying exchange across the alveolo-capillary barrier and transport by the blood can be grouped into four classes:- ú The ventilation of the lungs (the volume flow rate of gas) and the composition of the gas mixture to which mixed venous (pulmonary artery) blood will be exposed 9 Artificial Lung vs. Natural Lung § In the natural lungs, the factors underlying exchange across the alveolo-capillary barrier and transport by the blood can be grouped into four classes:- ú The permeability of the materials which separate the gas phase from the blood phase in the pulmonary alveoli 10 Artificial Lung vs. -

Left Atrial to Femoral Artery Full Cardiopulmonary Bypass: a Novel Technique for Descending and Thoracoabdominal Aortic Surgery
Published online: 2019-12-09 Original Article 19 Left Atrial to Femoral Artery Full Cardiopulmonary Bypass: A Novel Technique for Descending and Thoracoabdominal Aortic Surgery Dimitra Papanikolaou, MD1 Chris Savio, BS, CCP1 Mohammad A. Zafar, MBBS1 Leon Freudzon, MD1 Jinlin Wu, MD1 Mohamed Abdelbaky, MD1 Keith J. Pelletier, MBA, MHS, CCP1 Joelle Buntin, MSN, RN, RN-BC1 Thais Faggion Vinholo, BS, MSc1 Bulat A. Ziganshin, MD, PhD1,2 Brian Schwartz, MS, CCP1 John A. Elefteriades, MD, PhD (Hon)1 1 Aortic Institute at Yale-New Haven Hospital, Yale University School Address for correspondence John A. Elefteriades, MD, Aortic Institute of Medicine, New Haven, Connecticut at Yale-New Haven, Yale University School of Medicine, Clinic Building 2 Department of Surgical Diseases, Kazan State Medical University, CB 317, 789 Howard Avenue, New Haven, CT 06519 Kazan, Russia (e-mail: [email protected]). Int J Angiol 2020;29:19–26. Abstract Left atrial-femoral artery (LA-FA) bypass with a centrifugal pump and no oxygenator is commonly used for descending and thoracoabdominal aortic (DTAA) operations, mitigating the deleterious effects of cross-clamping. We present our initial experience performing DTAA replacement under LA-FA (left-to-left) cardiopulmonary bypass (CPB) with an oxygenator. DTAA replacement under LA-FA bypass with an oxygenator was performed in 14 consecutive patients (CPB group). The pulmonary vein and femoral artery (or distal aorta) were cannulated and the full CPB machine were used, including oxygenator, roller pump, pump suckers, and kinetically enhanced drainage. The CPB group was compared with 50 consecutive patients who underwent DTAA replacement utilizing traditional LA-FA bypass without an oxygenator (LA-FA group). -

280 Total Artificial Hearts and Implantable Ventricular Assist
Medical Policy Total Artificial Hearts and Implantable Ventricular Assist Devices Table of Contents • Policy: Commercial • Coding Information • Information Pertaining to All Policies • Policy: Medicare • Description • References • Authorization Information • Policy History Policy Number: 280 BCBSA Reference Number: 7.03.11 Related Policies • Heart/Lung Transplant, #269 • Heart Transplant, #197 • Extracorporeal Membrane Oxygenation, #726 Policy Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Bridge to Transplantation Implantable ventricular assist devices (VADs) with Food and Drug Administration (FDA) approval or clearance may be considered MEDICALLY NECESSARY as a bridge to heart transplantation for patients who are currently listed as heart transplantation candidates and not expected to survive until a donor heart can be obtained, or are undergoing evaluation to determine candidacy for heart transplantation. Implantable (VADs) with FDA approval or clearance, including humanitarian device exemptions, may be considered MEDICALLY NECESSARY as a bridge to heart transplantation in children 16 years old or younger who are currently listed as heart transplantation candidates and not expected to survive until a donor heart can be obtained, or are undergoing evaluation to determine candidacy for heart transplantation. Total artificial hearts (TAHs) with FDA-approved devices may be considered MEDICALLY NECESSARY as a bridge to heart transplantation for patients with biventricular failure who have no other reasonable medical -

Total Artificial Heart and Ventricular Assist Devices
UnitedHealthcare® Commercial Medical Policy Total Artificial Heart and Ventricular Assist Devices Policy Number: 2021T0384V Effective Date: May 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policy Coverage Rationale ........................................................................... 1 • Clinical Trials Documentation Requirements......................................................... 1 Applicable Codes .............................................................................. 2 Community Plan Policy Description of Services ..................................................................... 2 • Total Artificial Heart and Ventricular Assist Devices Clinical Evidence ............................................................................... 2 Medicare Advantage Coverage Summary U.S. Food and Drug Administration ................................................ 5 • Ventricular Assist Device (VAD) and Artificial Heart References ......................................................................................... 6 Policy History/Revision Information................................................ 7 Related Clinical Guidelines Instructions for Use ........................................................................... 8 • Mechanical Circulatory Support Devices (MCSD) • Transplant Review Guidelines: Solid Organ Transplantation Coverage Rationale The SynCardia™ temporary Total Artificial Heart is proven and medically necessary as a bridge to heart transplantation in members who meet all of -

Total Artificial Heart Reference Number: CP.MP.127 Coding Implications Last Review Date: 11/20 Revision Log
CEN,:'ENS:" ~·orporalion Clinical Policy: Total Artificial Heart Reference Number: CP.MP.127 Coding Implications Last Review Date: 11/20 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The SynCardia temporary Total Artificial Heart (TAH) (SynCardia Systems Inc.), formerly known as the CardioWest Total Artificial Heart, is a biventricular pulsatile pump that replaces the patient’s native ventricles and valves. This policy describes the medical necessity requirements for the total artificial heart. Policy/Criteria I. It is the policy of health plans affiliated with Centene Corporation® that the TAH is medically necessary as a bridge to heart transplantation when all of the following criteria are met: A. Member/enrollee is approved for cardiac transplant and is currently on transplant list; B. New York Heart Association (NYHA) Functional Class IV; C. Presence of non-reversible biventricular failure unresponsive to all other treatments; D. Ineligible for other ventricular support devices; E. Compatible donor heart is currently unavailable; F. Imminent risk of death; G. The device is U.S. FDA approved and used according to the FDA-labeled indications, contraindications, warnings and precautions; H. Member/enrollee is able to receive adequate anti-coagulation while on the total artificial heart. II. It is the policy of health plans affiliated with Centene Corporation that the TAH is experimental/investigational for use as destination therapy (permanent replacement of the failing heart). III. It is the policy of health plans affiliated with Centene Corporation that hospital discharge of members/enrollees implanted with the TAH who are supported by portable drivers (e.g., the Freedom portable driver) is experimental/investigational. -

The Mayo Gibbon Heart-Lung Bypass Machine in the Marc
Mayo Clinic Proceedings Legacy December 2014 Pioneers in Cardiac Surgery: The Mayo Gibbon Heart-Lung Bypass Machine This preliminary report shared the results from animal studies, but by May of that same year, Mayo Clinic had initiated use of the apparatus for human subjects and reported on the outcome in 8 cases.2 These patients had the following conditions: (1) ventricular septal defect, (2) Tetralogy of Fallot, or (3) persistent common atrioventricular canal. Four of the patients survived the surgery, one died shortly after the procedure, two died 3 hours postoperatively, and one died 6 days postoperatively.2 Mayo Clinic continued to study and develop successful surgical treatments in cardiology throughout the ensuing years. More than 60 years have passed since these first attempts, and success in cardiac surgery is directly related to these courageous research pioneers and patients. 1,2,3 This historical line of research in In the March 13, 1955, issue of The Proceedings cardiology also represents another successful of the Staff Meetings of the Mayo Clinic, Dr collaboration between the field of medicine John W. Kirklin and colleagues shared a and industry. International Business Machines preliminary report of their research from the (IBM) aided in the design and development of previous 3 years which tackled the challenges the Mayo Gibbon Heart-Lung Bypass of cardiac surgery. While other types of Machine.1,2,3 surgeries have their own impediments, cardiac surgery is dominated by the need to keep a References patient alive during the procedure while 1. Jones RE, Donald DE, Swan HJ, Harshbarger HG, maintaining the function of the circulatory Kirklin JW, Wood EH. -

Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Operation
Thoracic Key Fastest Thoracic Insight Engine Home Log In Register Categories » More References » About Gold Membership Contact Search... Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Operation Principles of Oxygenator Function: Gas Exchange, Heat Transfer, and Operation Michael H. Hines INTRODUCTION Since the early 1950s when the development of a heart-lung machine first began, there has been a tremendous evolution of devices and machinery for cardiac support (1,2). However, despite the diversity in designs through the years, they all contain three essential components: a mechanism to circulate the blood, a method of gas exchange for oxygen and carbon dioxide, and some mechanism for temperature control. Chapter 2 has covered the first important component, and we will now focus on the two subsequent elements: gas exchange and heat transfer. And while it is referred to as the “oxygenator,” we must recognize that it is responsible for the movement of both oxygen in, as well as carbon dioxide out. The discussion will start with a basic review of the principles of physics, and then we will apply those principles to the devices used specifically in extracorporeal support, including cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO). You may notice as you go through this chapter that there is a scarcity of trade and manufacturer names. The author has intentionally avoided using these. The intent was primarily to focus on the physiology, physics, and chemistry of the oxygenator and heat exchanger, but also to emphasize the fact that there are a large number of manufacturers producing many products that have all been shown to function extremely well. -
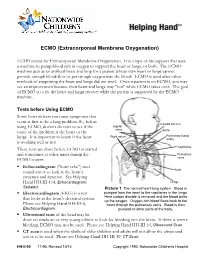
ECMO (Extracorporeal Membrane Oxygenation)
ECMO (Extracorporeal Membrane Oxygenation) ECMO stands for Extracorporeal Membrane Oxygenation. It is a type of life support that uses a machine to pump blood rich in oxygen to support the heart or lungs, or both. The ECMO machine acts as an artificial heart and lung for a patient whose own heart or lungs cannot provide enough blood flow or get enough oxygen into the blood. ECMO is used after other methods of supporting the heart and lungs did not work. Once a patient is on ECMO, you may see an improvement because their heart and lungs may "rest" while ECMO takes over. The goal of ECMO is to let the heart and lungs recover while the patient is supported by the ECMO machine. Tests before Using ECMO Some heart defects can cause symptoms that seem at first to be a lung problem. So, before Carotid arteries using ECMO, doctors do tests to see if the Jugular veins cause of the problem is the heart or the Aorta Right atrium Pulmonary (lung) lungs. It is important to know if the heart artery is working well or not. Capillaries These tests are done before ECMO is started and sometimes at other times during the Pulmonary ECMO course: veins . Echocardiogram ("heart echo") uses Heart sound waves to look at the heart’s structure and function. See Helping Hand HH-III-114, Echocardiogram: Lungs Sedated. Picture 1 The normal heart-lung system. Blood is . Electrocardiogram (EKG) is a test pumped from the heart to the capillaries in the lungs. that looks at the heart's electrical system. -

Directions for Use Tandemlung Oxygenator CAT# 5160-0000
Directions for Use TandemLung Oxygenator CAT# 5160-0000 7000-0161 Rev.7 2018-08-23 Page | 2 Figure 1. TandemLung Oxygenator Page | 3 1. Overview The TandemLung Oxygenator is used in cardiac surgery, in combination with a blood pump to oxygenate blood and remove carbon dioxide. The device consists of a hollow fiber membrane with blood inflow and outflow port and gas inlet and outlet port. Blood enters into the center of the fiber bundle where it is distributed radially and uniformly across the fiber bundle by a conical diffuser. The compact nature of the device, the orientation of the inflow/outflow ports, and optimized gap sizes on the inlet and outlet of the bundle simplify the priming process. 2. Indications for Use The TandemLung Oxygenator (TLO) is intended to be used for adult patients for extracorporeal circulation during cardiopulmonary bypass in the field of open-heart surgery. Within the indicated flow rates blood is oxygenated and carbon dioxide is removed. The utilization period of this device is restricted to six hours. 3. Contraindications No contraindications are known provided the device is used within the Indications for Use and in accordance with the stated operating conditions. 4. Warnings and Precautions Only use the device in accordance with these Instructions for Use. • The oxygenator may only be used by persons trained in extracorporeal circulation procedures. • Possible side effects include infections, hemolysis, and embolisms. Side effects associated with extracorporeal circulation in general may also occur (e.g., post- perfusion syndrome and organ damage). • Observe the use-by date on the device packaging.