From Four- to Three-Segmented Labium in Reduviidae (Hemiptera: Heteroptera)*)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insetos Do Brasil
COSTA LIMA INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS A. DA COSTA LIMA Professor Catedrático de Entomologia Agrícola da Escola Nacional de Agronomia Ex-Chefe de Laboratório do Instituto Oswaldo Cruz INSETOS DO BRASIL 2.º TOMO CAPÍTULO XXII HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 CONTEUDO CAPÍTULO XXII PÁGINA Ordem HEMÍPTERA ................................................................................................................................................ 3 Superfamília SCUTELLEROIDEA ............................................................................................................ 42 Superfamília COREOIDEA ............................................................................................................................... 79 Super família LYGAEOIDEA ................................................................................................................................. 97 Superfamília THAUMASTOTHERIOIDEA ............................................................................................... 124 Superfamília ARADOIDEA ................................................................................................................................... 125 Superfamília TINGITOIDEA .................................................................................................................................... 132 Superfamília REDUVIOIDEA ........................................................................................................................... -

British Museum (Natural History)
Bulletin of the British Museum (Natural History) Darwin's Insects Charles Darwin 's Entomological Notes Kenneth G. V. Smith (Editor) Historical series Vol 14 No 1 24 September 1987 The Bulletin of the British Museum (Natural History), instituted in 1949, is issued in four scientific series, Botany, Entomology, Geology (incorporating Mineralogy) and Zoology, and an Historical series. Papers in the Bulletin are primarily the results of research carried out on the unique and ever-growing collections of the Museum, both by the scientific staff of the Museum and by specialists from elsewhere who make use of the Museum's resources. Many of the papers are works of reference that will remain indispensable for years to come. Parts are published at irregular intervals as they become ready, each is complete in itself, available separately, and individually priced. Volumes contain about 300 pages and several volumes may appear within a calendar year. Subscriptions may be placed for one or more of the series on either an Annual or Per Volume basis. Prices vary according to the contents of the individual parts. Orders and enquiries should be sent to: Publications Sales, British Museum (Natural History), Cromwell Road, London SW7 5BD, England. World List abbreviation: Bull. Br. Mus. nat. Hist. (hist. Ser.) © British Museum (Natural History), 1987 '""•-C-'- '.;.,, t •••v.'. ISSN 0068-2306 Historical series 0565 ISBN 09003 8 Vol 14 No. 1 pp 1-141 British Museum (Natural History) Cromwell Road London SW7 5BD Issued 24 September 1987 I Darwin's Insects Charles Darwin's Entomological Notes, with an introduction and comments by Kenneth G. -

Good Water Ripples Volume 7 Number 4
For information contact: http://txmn.org/goodwater [email protected] Volume 7 Number 4 August/September 2018 Editor: Mary Ann Melton Fall Training Class Starts Soon Good Water Mas- ter Naturalist Fall Training Class will start Tuesday even- ing, September 4th. The class will meet UPCOMING EVENTS on Tuesday eve- nings from 6:00- 8/9/18 NPSOT 9:30 p.m. Some 8/13/18 WAG classes and field trips will be on Sat- 8/23/18 GWMN urdays. The first class is Tuesday, Austin Butterfly Forum 8/27/18 September 4. The 9/5/18 NPAT last class will be December 11. Cost is $150 and includes the comprehensive Texas Master 9/13/18 NPSOT Naturalist Program manual as well as a one year membership to the Good 9/20/18 Travis Audubon Water Chapter. For couples who plan to share the manual, there is a dis- count for the second student. 9/24/18 Austin Butterfly Forum Click here for online registration. The Tuesday classes will start at 6:00 9/27/18 GWMN p.m. and finish around 9:30. There are four Saturday field trips and classes planned. The schedule will be posted in the next week or so. Check back Check the website for additional here after August 15 for the link to the schedule. events including volunteer and training opportunities. The events Click here: https://txmn.org/goodwater/Training-class-online-application/ are too numerous to post here. for Online Training Registration David Robinson took our Spring Training Class this year. He says, "The Fall Training Class Starts Soon 1 Instructors & Speakers were absolutely fantastic. -

New Evidence for the Presence of the Telomere Motif (TTAGG)N in the Family Reduviidae and Its Absence in the Families Nabidae
COMPARATIVE A peer-reviewed open-access journal CompCytogen 13(3): 283–295 (2019)Telomere motif (TTAGG ) in Cimicomorpha 283 doi: 10.3897/CompCytogen.v13i3.36676 RESEARCH ARTICLEn Cytogenetics http://compcytogen.pensoft.net International Journal of Plant & Animal Cytogenetics, Karyosystematics, and Molecular Systematics New evidence for the presence of the telomere motif (TTAGG) n in the family Reduviidae and its absence in the families Nabidae and Miridae (Hemiptera, Cimicomorpha) Snejana Grozeva1, Boris A. Anokhin2, Nikolay Simov3, Valentina G. Kuznetsova2 1 Cytotaxonomy and Evolution Research Group, Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia 1000, 1 Tsar Osvoboditel, Bulgaria2 Department of Karyosystematics, Zoological Institute, Russian Academy of Sciences, St. Petersburg 199034, Universitetskaya nab., 1, Russia 3 National Museum of Natural History, Bulgarian Academy of Sciences, Sofia 1000, 1 Tsar Osvoboditel, Bulgaria Corresponding author: Snejana Grozeva ([email protected]) Academic editor: M. José Bressa | Received 31 May 2019 | Accepted 29 August 2019 | Published 20 September 2019 http://zoobank.org/9305DF0F-0D1D-44FE-B72F-FD235ADE796C Citation: Grozeva S, Anokhin BA, Simov N, Kuznetsova VG (2019) New evidence for the presence of the telomere motif (TTAGG)n in the family Reduviidae and its absence in the families Nabidae and Miridae (Hemiptera, Cimicomorpha). Comparative Cytogenetics 13(3): 283–295. https://doi.org/10.3897/CompCytogen.v13i3.36676 Abstract Male karyotype and meiosis in four true bug species belonging to the families Reduviidae, Nabidae, and Miridae (Cimicomorpha) were studied for the first time using Giemsa staining and FISH with 18S ribo- somal DNA and telomeric (TTAGG)n probes. We found that Rhynocoris punctiventris (Herrich-Schäffer, 1846) and R. -

NOTES on WATER BUGS from SOUTH EAST ASIA and AUSTRALIA (Heteroptera: Nepomorpha & Gerromorpha)
F. M. BUZZETTI, N. NIESER & J. DAMGAARD: Notes on water bugs ... 31 FILIPPO MARIA BUZZETTI, NICO NIESER & JAKOB DAMGAARD NOTES ON WATER BUGS FROM SOUTH EAST ASIA AND AUSTRALIA (Heteroptera: Nepomorpha & Gerromorpha) ABSTRACT - BUZZETTI F.M., NIESER N. & DAMGAARD J., 2006 - Notes on water bugs from South East Asia and Australia (Heteroptera: Nepomorpha & Gerromorpha). Atti Acc. Rov. Agiati, a. 256, 2006, ser. VIII, vol. VI, B: 31-45. Faunistical data on some Nepomorpha and Gerromorpha from South East Asia and Australia are given. Hydrometra greeni Kirk., Limnogonus (Limnogonoides) pecto- ralis Mayr and Halobates sp. are reported as new records for Myanmar. KEY WORDS - Faunistics. RIASSUNTO - BUZZETTI F.M., NIESER N. & DAMGAARD J., 2006 - Su alcuni Emitteri acquatici del Sud Est Asiatico e dellAustralia (Heteroptera: Nepomorpha & Gerro- morpha). Si riportano alcuni dati faunistici relativi a Nepomorfi e Gerromorfi dal Sud Est Asiatico e dallAustralia. Hydrometra greeni Kirk., Limnogonus (Limnogonoides) pecto- ralis Mayr e Halobates sp. sono per la prima volta citati per il Myanmar. PAROLE CHIAVE - Faunistica. INTRODUCTION In this publication the Nepomorpha and Gerromorpha from South East Asia and Australia presently in the F. M. B. private collection are reported. Some specimens transferred to the Nieser collection are indi- cated NCTN. Other data from the collection of the Zoological Muse- um of Copenhagen University are indicated as ZMUC. Some synony- my is abbraviated but can be found in the publications cited under the various species. When given, measurements are in mm. The collecting localities from Myanmar are shown in Map 1. 32 Atti Acc. Rov. Agiati, a. 256, 2006, ser. -

Addenda to the Insect Fauna of Al-Baha Province, Kingdom of Saudi Arabia with Zoogeographical Notes Magdi S
JOURNAL OF NATURAL HISTORY, 2016 VOL. 50, NOS. 19–20, 1209–1236 http://dx.doi.org/10.1080/00222933.2015.1103913 Addenda to the insect fauna of Al-Baha Province, Kingdom of Saudi Arabia with zoogeographical notes Magdi S. El-Hawagrya,c, Mostafa R. Sharafb, Hathal M. Al Dhaferb, Hassan H. Fadlb and Abdulrahman S. Aldawoodb aEntomology Department, Faculty of Science, Cairo University, Giza, Egypt; bPlant Protection Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, Kingdom of Saudi Arabia; cSurvey and Classification of Agricultural and Medical Insects in Al-Baha Province, Al-Baha University, Al-Baha, Saudi Arabia ABSTRACT ARTICLE HISTORY The first list of insects (Arthropoda: Hexapoda) of Al-Baha Received 1 April 2015 Province, Kingdom of Saudi Arabia (KSA) was published in 2013 Accepted 30 September 2015 and contained a total of 582 species. In the present study, 142 Online 9 December 2015 species belonging to 51 families and representing seven orders KEYWORDS are added to the fauna of Al-Baha Province, bringing the total Palaearctic; Afrotropical; number of species now recorded from the province to 724. The Eremic; insect species; reported species are assigned to recognized regional zoogeogra- Arabian Peninsula; Tihama; phical regions. Seventeen of the species are recorded for the first Al-Sarah; Al-Sarawat time for KSA, namely: Platypleura arabica Myers [Cicadidae, Mountains Hemiptera]; Cletomorpha sp.; Gonocerus juniperi Herrich-Schäffer [Coreidae, Hemiptera]; Coranus lateritius (Stål); Rhynocoris bipus- tulatus (Fieber) [Reduviidae, Hemiptera]; Cantacader iranicus Lis; Dictyla poecilla Drake & Hill [Tingidae, Hemiptera]; Mantispa scab- ricollis McLachlan [Mantispidae, Neuroptera]; Cerocoma schreberi Fabricius [Meloidae, Coleoptera]; Platypus parallelus (Fabricius) [Curculionidae, Coleoptera]; Zodion cinereum (Fabricius) [Conopidae, Diptera]; Ulidia ?ruficeps Becker [Ulidiidae, Diptera]; Atherigona reversura Villeneuve [Muscidae, Diptera]; Aplomya metallica (Wiedemann); Cylindromyia sp. -

Universidade Estadual Paulista “Júlio De Mesquita Filho” Faculdade De Ciências Agronômicas Campus De Botucatu Thaíse
UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” FACULDADE DE CIÊNCIAS AGRONÔMICAS CAMPUS DE BOTUCATU BIONOMIA E COMPORTAMENTO DE Atopozelus opsimus ELKINS (HEMIPTERA: REDUVIIDAE) MANTIDOS EM Glycaspis brimblecombei MOORE (HEMIPTERA: PSYLLIDAE) THAÍSE KARLA RIBEIRO DIAS Dissertação apresentada à Faculdade de Ciências Agronômicas da UNESP - Campus de Botucatu, para obtenção do título de Mestre em Agronomia - Proteção de Plantas. BOTUCATU - SP Fevereiro – 2009 UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” FACULDADE DE CIÊNCIAS AGRONÔMICAS CAMPUS DE BOTUCATU BIONOMIA E COMPORTAMENTO DE Atopozelus opsimus ELKINS (HEMIPTERA: REDUVIIDAE) MANTIDOS EM Glycaspis brimblecombei MOORE (HEMIPTERA: PSYLLIDAE) THAÍSE KARLA RIBEIRO DIAS Engenheira Agrônoma Orientador: Prof. Dr. Carlos Frederico Wilcken Dissertação apresentada à Faculdade de Ciências Agronômicas da UNESP - Campus de Botucatu, para obtenção do título de Mestre em Agronomia - Proteção de Plantas. BOTUCATU - SP Fevereiro - 2009 IV ! " ! $ ! % % & % V AGRADECIMENTOS A Faculdade de Ciências Agronômicas da Universidade Estadual Paulista-UNESP Campus de Botucatu por contribuir de forma impar para o desenvolvimento do país por meio da ciência. Ao Professor Dr. Carlos Frederico Wilcken por aceitar-me como sua orientada partilhar comigo seu conhecimento, seriedade, compromisso e respeito nesses dois anos. Além de educador, em minha vida "você" é mediador de um sonho. A Coordenadoria de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) pela concessão da bolsa de estudos. A todos os professores do Programa de Pós-graduação em Proteção de Plantas pelos ensinamentos. Ao Consórcio Paulista de Papel e Celulose – Conpacel, unidade de Boa Esperança do Sul, todo apoio durante as coletas e envio de material que deu origem a criação e manutenção dos percevejos em laboratório. -

Venom Composition and Bioactivity from the Eurasian Assassin Bug Rhynocoris Iracundus
biomedicines Article Hexapod Assassins’ Potion: Venom Composition and Bioactivity from the Eurasian Assassin Bug Rhynocoris iracundus Nicolai Rügen 1, Timothy P. Jenkins 2, Natalie Wielsch 3, Heiko Vogel 4 , Benjamin-Florian Hempel 5,6 , Roderich D. Süssmuth 5 , Stuart Ainsworth 7, Alejandro Cabezas-Cruz 8 , Andreas Vilcinskas 1,9,10 and Miray Tonk 9,10,* 1 Department of Bioresources, Fraunhofer Institute for Molecular Biology and Applied Ecology, Ohlebergsweg 12, 35392 Giessen, Germany; [email protected] (N.R.); [email protected] (A.V.) 2 Department of Biotechnology and Biomedicine, Technical University of Denmark, 2800 Kongens Lyngby, Denmark; [email protected] 3 Research Group Mass Spectrometry/Proteomics, Max Planck Institute for Chemical Ecology, Hans-Knoell-Strasse 8, 07745 Jena, Germany; [email protected] 4 Department of Entomology, Max Planck Institute for Chemical Ecology, Hans-Knöll-Straße 8, 07745 Jena, Germany; [email protected] 5 Department of Chemistry, Technische Universität Berlin, Strasse des 17. Juni 124, 10623 Berlin, Germany; [email protected] (B.-F.H.); [email protected] (R.D.S.) 6 BIH Center for Regenerative Therapies BCRT, Charité—Universitätsmedizin Berlin, 13353 Berlin, Germany 7 Centre for Snakebite Research and Interventions, Department of Tropical Disease Biology, Liverpool School of Tropical Medicine, Liverpool L3 5QA, UK; [email protected] 8 Citation: Rügen, N.; Jenkins, T.P.; UMR BIPAR, Laboratoire de Santé Animale, Anses, INRAE, Ecole Nationale Vétérinaire d’Alfort, Wielsch, N.; Vogel, H.; Hempel, B.-F.; F-94700 Maisons-Alfort, France; [email protected] 9 Institute for Insect Biotechnology, Justus Liebig University of Giessen, Heinrich-Buff-Ring 26-32, Süssmuth, R.D.; Ainsworth, S.; 35392 Giessen, Germany Cabezas-Cruz, A.; Vilcinskas, A.; 10 LOEWE Centre for Translational Biodiversity Genomics (LOEWE-TBG), Senckenberganlage 25, Tonk, M. -

A Comparison of the External Morphology and Functions of Labial Tip Sensilla in Semiaquatic Bugs (Hemiptera: Heteroptera: Gerromorpha)
Eur. J. Entomol. 111(2): 275–297, 2014 doi: 10.14411/eje.2014.033 ISSN 1210-5759 (print), 1802-8829 (online) A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) 1 2 JOLANTA BROŻeK and HERBERT ZeTTeL 1 Department of Zoology, Faculty of Biology and environmental Protection, University of Silesia, Bankowa 9, PL 40-007 Katowice, Poland; e-mail: [email protected] 2 Natural History Museum, entomological Department, Burgring 7, 1010 Vienna, Austria; e-mail: [email protected] Key words. Heteroptera, Gerromorpha, labial tip sensilla, pattern, morphology, function, apomorphic characters Abstract. The present study provides new data on the morphology and distribution of the labial tip sensilla of 41 species of 20 gerro- morphan (sub)families (Heteroptera: Gerromorpha) obtained using a scanning electron microscope. There are eleven morphologically distinct types of sensilla on the tip of the labium: four types of basiconic uniporous sensilla, two types of plate sensilla, one type of peg uniporous sensilla, peg-in-pit sensilla, dome-shaped sensilla, placoid multiporous sensilla and elongated placoid multiporous sub- apical sensilla. Based on their external structure, it is likely that these sensilla are thermo-hygrosensitive, chemosensitive and mechano- chemosensitive. There are three different designs of sensilla in the Gerromorpha: the basic design occurs in Mesoveliidae and Hebridae; the intermediate one is typical of Hydrometridae and Hermatobatidae, and the most specialized design in Macroveliidae, Veliidae and Gerridae. No new synapomorphies for Gerromorpha were identified in terms of the labial tip sensilla, multi-peg structures and shape of the labial tip, but eleven new diagnostic characters are recorded for clades currently recognized in this infraorder. -

Heteroptera, Reduviidae, Harpactorinae) *
Redescription of theS. Grozeva Neotropical & genusN. Simov Aristathlus (Eds) (Heteroptera, 2008 Reduviidae, Harpactorinae) 85 ADVANCES IN HETEROPTERA RESEARCH Festschrift in Honour of 80th Anniversary of Michail Josifov, pp. 85-103. © Pensoft Publishers Sofi a–Moscow Redescription of the Neotropical genus Aristathlus (Heteroptera, Reduviidae, Harpactorinae) * D. Forero1, H.R. Gil-Santana2 & P.H. van Doesburg3 1 Division of Invertebrate Zoology (Entomology), American Museum of Natural History, New York, New York 10024–5192; and Department of Entomology, Comstock Hall, Cornell University, Ithaca, New York 14853–2601, USA. E-mail: [email protected] 2 Departamento de Entomologia, Instituto Oswaldo Cruz, Avenida Brasil 4365, Rio de Janeiro, 21045-900, Brazil. E-mail: [email protected] 3 Nationaal Natuurhistorisch Museum, Postbus 9517, 2300 RA Leiden, Th e Netherlands. E-mail: [email protected] ABSTRACT Th e Neotropical genus Aristathlus Bergroth 1913, is redescribed. Digital dorsal habitus photographs for A. imperatorius Bergroth and A. regalis Bergroth, the two included species, are provided. Selected morphological structures are documented with scanning electron micrographs. Male genitalia are documented for the fi rst time with digital photomicrographs and line drawings. New distributional records in South America are given for species of Aristathlus. Keywords: Harpactorini, Hemiptera, male genitalia, Neotropical region, taxonomy. INTRODUCTION Reduviidae is the second largest family of Heteroptera with more than 6000 species described (Maldonado 1990). Despite not having an agreement about the suprageneric classifi cation of Reduviidae (e.g., Putshkov & Putshkov 1985; Maldonado 1990), * Th is paper is dedicated to Michail Josifov on the occasion of his 80th birthday. 86 D. Forero, H.R. Gil-Santana & P.H. -

The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V
The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V. Bennett Edwin F. Cook Technical Bulletin 332-1981 Agricultural Experiment Station University of Minnesota St. Paul, Minnesota 55108 CONTENTS PAGE Introduction ...................................3 Key to Adults of Nearctic Families of Semiaquatic Hemiptera ................... 6 Family Saldidae-Shore Bugs ............... 7 Family Mesoveliidae-Water Treaders .......18 Family Hebridae-Velvet Water Bugs .......20 Family Hydrometridae-Marsh Treaders, Water Measurers ...22 Family Veliidae-Small Water striders, Rime bugs ................24 Family Gerridae-Water striders, Pond skaters, Wherry men .....29 Family Ochteridae-Velvety Shore Bugs ....35 Family Gelastocoridae-Toad Bugs ..........36 Literature Cited ..............................37 Figures ......................................44 Maps .........................................55 Index to Scientific Names ....................59 Acknowledgement Sincere appreciation is expressed to the following individuals: R. T. Schuh, for being extremely helpful in reviewing the section on Saldidae, lending specimens, and allowing use of his illustrations of Saldidae; C. L. Smith for reading the section on Veliidae, checking identifications, and advising on problems in the taxon omy ofthe Veliidae; D. M. Calabrese, for reviewing the section on the Gerridae and making helpful sugges tions; J. T. Polhemus, for advising on taxonomic prob lems and checking identifications for several families; C. W. Schaefer, for providing advice and editorial com ment; Y. A. Popov, for sending a copy ofhis book on the Nepomorpha; and M. C. Parsons, for supplying its English translation. The University of Minnesota, including the Agricultural Experi ment Station, is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, creed, color, sex, national origin, or handicap. The information given in this publication is for educational purposes only. -
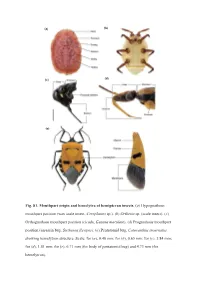
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V