Preliminary Survey Work Fails to Detect the Amphibian Chytrid Pathogen In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Africa's Gulf of Guinea Forests: Biodiversity Patterns and Conservation Priorities
Advances in Applied Biodiversity Science, no. 6 AABSAdvances in Applied Biodiversity Science Number 6 Africa’s Gulf of Guinea Forests: Africa’s Gulf of Guinea Forests:Biodiversity Patterns and Conservation Africa’s Biodiversity Patterns and Conservation Priorities John F. Oates, Richard A. Bergl, and Joshua M. Linder Priorities C Conservation International ONSERVATION 1919 M Street, NW, Suite 600 Washington, DC 20036 TEL: 202-912-1000 FAX: 202-912-0772 I NTERNATIONAL ISBN 1-881173-82-8 WEB: www.conservation.org 9 0 0 0 0> www.biodiversityscience.org 9781881173823 About the Authors John F. Oates is a CABS Research Fellow, Professor of Anthropology at Hunter College, City University of New York (CUNY), and a Senior Conservation Advisor to the Africa program of the Wildlife Conservation Society (WCS). He is cur- rently advising WCS on biodiversity conservation projects in eastern Nigeria and western Cameroon. Dr. Oates has conducted research on the ecology of forest primates in Africa and Asia since 1966, and has assisted with the development of rainforest protected areas in South India and West Africa. He has published extensively on primate biology and conservation and, as an active member of the IUCN-SSC Primate Specialist Group, has compiled conservation action plans for African primates. He holds a PhD from the University of London. Richard A. Bergl is a doctoral student in anthropology at the CUNY Graduate Center, in the graduate training program of the New York Consortium in Evolutionary Primatology (NYCEP). He is currently conducting research into the population and habitat viability of the Cross River gorilla (Gorilla gorilla diehli) in Nigeria and Cameroon. -

2008 Board of Governors Report
American Society of Ichthyologists and Herpetologists Board of Governors Meeting Le Centre Sheraton Montréal Hotel Montréal, Quebec, Canada 23 July 2008 Maureen A. Donnelly Secretary Florida International University Biological Sciences 11200 SW 8th St. - OE 167 Miami, FL 33199 [email protected] 305.348.1235 31 May 2008 The ASIH Board of Governor's is scheduled to meet on Wednesday, 23 July 2008 from 1700- 1900 h in Salon A&B in the Le Centre Sheraton, Montréal Hotel. President Mushinsky plans to move blanket acceptance of all reports included in this book. Items that a governor wishes to discuss will be exempted from the motion for blanket acceptance and will be acted upon individually. We will cover the proposed consititutional changes following discussion of reports. Please remember to bring this booklet with you to the meeting. I will bring a few extra copies to Montreal. Please contact me directly (email is best - [email protected]) with any questions you may have. Please notify me if you will not be able to attend the meeting so I can share your regrets with the Governors. I will leave for Montréal on 20 July 2008 so try to contact me before that date if possible. I will arrive late on the afternoon of 22 July 2008. The Annual Business Meeting will be held on Sunday 27 July 2005 from 1800-2000 h in Salon A&C. Please plan to attend the BOG meeting and Annual Business Meeting. I look forward to seeing you in Montréal. Sincerely, Maureen A. Donnelly ASIH Secretary 1 ASIH BOARD OF GOVERNORS 2008 Past Presidents Executive Elected Officers Committee (not on EXEC) Atz, J.W. -

FROGLOG Newsletter of the IUCN /SSC Amphibian Specialist Group (ASG)
Pyxicephalus adspersus byTim Halliday ISSN 1026-0269 FROGLOG Newsletter of the IUCN /SSC Amphibian Specialist Group (ASG) February 2007, Number 79 to cause mass mortality. Kevin but is under continuous threat from Amphibian Declines in Africa Smith reported studies of stream a variety of factors, including water By Tim Halliday frogs in the Drakensburg extraction and climate change. The 8th meeting of the where mortality does occur, at Because its tadpoles take more Herpetological Association of higher altitudes, in Strongylopus than a year to reach metamorpho- Africa (HAA) was held in hymenopus, but the impact of this sis, it is very dependent on Potchefstroom, South Africa from on populations is not yet clear. perennial streams. John Measey 24 to 27 November, 2006. The He also reported that chytrid- described his work on the meeting, sponsored by the DAPTF, infected tadpoles of Heleophryne landscape genetics of the Kenyan was organised by Louis du Preez. natalensis develop distinctive frog Schoutedenella xenodacty- It provided a very interesting markings around their mouthparts; loides which, surprisingly, suggests overview of work on amphibians no mass mortality in this species that this tiny frog can disperse long currently going on in southern has been observed. distances across unsuitable dry Africa, including a number of The African bullfrog savannah. projects funded by the DAPTF. Pyxicephalus adspersus is Ernst Baard reported on recent In a workshop on amphibian threatened by accelerating habitat changes in legislation and policy in declines, Les Minter reviewed the loss in many parts of its range; South Africa with regard to status of southern African while many breeding sites are protected, threatened, alien and amphibians; 20 endemic species protected, less attention has been invasive species, and was are endangered, and habitat paid to its terrestrial habitat. -

A Survey of Amphibians and Reptiles in the Foothills of Mount Kupe, Cameroon
Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 10(2) [Special Section]: 37–67 (e131). A survey of amphibians and reptiles in the foothills of Mount Kupe, Cameroon 1,2Daniel M. Portik, 3,4Gregory F.M. Jongsma, 3Marcel T. Kouete, 3Lauren A. Scheinberg, 3Brian Freiermuth, 5,6Walter P. Tapondjou, and 3,4David C. Blackburn 1Museum of Vertebrate Zoology, University of California, Berkeley, 3101 Valley Life Sciences Building, Berkeley, California 94720, USA 2Department of Biology, University of Texas at Arlington, 501 S. Nedderman Drive, Box 19498, Arlington, Texas 76019-0498, USA 3California Academy of Sciences, San Francisco, California 94118, USA 4Florida Museum of Natural History, University of Florida, Gainesville, Florida 32611, USA 5Laboratory of Zoology, Faculty of Science. University of Yaoundé, PO Box 812 Yaoundé, Cameroon, AFRICA 6Department of Ecology and Evolutionary Biology, University of Kansas, 1450 Jayhawk Boulevard, Lawrence, Kansas 66045, USA Abstract.—We performed surveys at several lower elevation sites surrounding Mt. Kupe, a mountain at the southern edge of the Cameroonian Highlands. This work resulted in the sampling of 48 species, including 38 amphibian and 10 reptile species. By combining our data with prior survey results from higher elevation zones, we produce a checklist of 108 species for the greater Mt. Kupe region including 72 frog species, 21 lizard species, and 15 species of snakes. Our work adds 30 species of frogs at lower elevations, many of which are associated with breeding in pools or ponds that are absent from the slopes of Mt. Kupe. We provide taxonomic accounts, including museum specimen data and associated molecular data, for all species encountered. -

Are There Shifts in Amphibian Faunal Composition in Nigerian Landscapes Undergoing Long-Term Degradation? a Case Study from a Montane Environment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by I-Revues ARE THERE SHIFTS IN AMPHIBIAN FAUNAL COMPOSITION IN NIGERIAN LANDSCAPES UNDERGOING LONG-TERM DEGRADATION? A CASE STUDY FROM A MONTANE ENVIRONMENT Jerry M. LEA1, Luca LUISELLI2 & Edoardo POLITANO2 RÉSUMÉ. — Quelles variations subit la composition du peuplement d’Amphibiens dans les paysa- ges nigériens subissant une dégradation sur le long terme ? Le cas d’un environnement de montagne. — Les forêts tropicales mondiales subissent une importante altération anthropique. Les données recueillies au cours de diverses études dans les forêts du Nigéria montrent que l’herpétofaune subit ces modifica- tions d’habitat. En particulier, quand les environnements forestiers tropicaux se dégradent et bien que la diversité globale reste stable ou voire même augmente, on remarque que l’on passe d’une dominance d’espèces spécialistes forestières à une dominance d’espèces généralistes capables d’utiliser une large gamme d’habitats. La même modification s’observe quels que soient le type d’habitat (e. g. forêt basse temporairement inondée ou forêt de montagne) ou le taxon (e. g. anoures ou serpents). Un inventaire des amphibiens a été conduit en 2002 sur le plateau d’Obudu au Nigéria, qui est une zone d’intérêt majeur pour la conservation, abritant beaucoup d’espèces endémiques et menacées. L’environnement y a été gravement dégradé et a souffert d’une forte déforestation dans les années récentes ; le tourisme de plai- sance, en pleine extension, menace d’avoir un impact encore plus grand. En comparant les résultats de cet inventaire à divers jeux de données des années 1960 et 1980, nous sommes en mesure de décrire les changements dans la composition des peuplements intervenus après de nombreuses années de perturba- tion anthropique et nous discutons les raisons du succès ou de l’échec d’espèces spécialistes ou généra- listes particulières. -

Litoria Wilcoxii)
Behavioural Ecology, Reproductive Biology and Colour Change Physiology in the Stony Creek Frog (Litoria wilcoxii) Author Kindermann, Christina Published 2017 Thesis Type Thesis (PhD Doctorate) School Griffith School of Environment DOI https://doi.org/10.25904/1912/1098 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/367513 Griffith Research Online https://research-repository.griffith.edu.au Behavioural ecology, reproductive biology and colour change physiology in the Stony Creek Frog (Litoria wilcoxii) Christina Kindermann B. Sc. (Hons) Griffith University School of Environment Environmental Futures Research Institute Submitted in fulfilment of the requirements of the degree of Doctor of Philosophy July 2016 Abstract Many animals possess the remarkable ability to change their skin colour. Colour change can have several potential functions, including communication, thermoregulation and camouflage. However, while the physiological mechanisms and functional significance of colour change in other vertebrates have been well studied, the role of colour change in amphibians is still relatively unknown and a disconnection between morphology, physiology and function exists in the literature (review presented in chapter 2). In this thesis, I investigate these multidisciplinary components to understand the processes and functions of colour change in stony creek frogs (Litoria wilcoxii), which are known to turn bright yellow during the breeding season. By (1 – Chapter 3) examining the distribution and structure of dermal pigment cells, (2– Chapter 4) determining hormonal triggers of rapid colour change, (3– Chapter 5) investigating seasonal colour, hormone and disease relationships and (4– Chapter 6) determining the evolutionary functions of colour change, I provide a comprehensive explanation of this phenomenon in L. -

ABSTRACTS 29 Reptile Ecology I, Highland A, Sunday 15 July 2018
THE JOINT MEETING OF ASIH SSAR HL lcHTHYOLOGISTS & HERPETOLOGISTS ROCHESTER, NEW YORK 2018 ABSTRACTS 29 Reptile Ecology I, Highland A, Sunday 15 July 2018 Curtis Abney, Glenn Tattersall and Anne Yagi Brock University, St. Catharines, Ontario, Canada Thermal Preference and Habitat Selection of Thamnophis sirtalis sirtalis in a Southern Ontario Peatland Gartersnakes represent the most widespread reptile in North America. Despite occupying vastly different biogeoclimatic zones across their range, evidence suggests that the thermal preferenda (Tset) of gartersnakes has not diverged significantly between populations or different Thamnophis species. The reason behind gartersnake success could lie in their flexible thermoregulatory behaviours and habitat selection. We aimed to investigate this relationship by first identifying the Tset of a common gartersnake species (Thamnophis sirtalis sirtalis) via a thermal gradient. We then used this Tset parameter as a baseline for calculating the thermal quality of an open, mixed, and forested habitat all used by the species. We measured the thermal profiles of these habitats by installing a series of temperature-recording analogues that mimicked the reflectance and morphology of living gartersnakes and recorded environmental temperatures as living snakes experience them. Lastly, we used coverboards to survey the current habitat usage of T. s. sirtalis. Of the three habitats, we found that the open habitat offered the highest thermal quality throughout the snake’s active season. In contrast, we recorded the greatest number of snakes using the mixed habitat which had considerably lower thermal quality. Although the open habitat offered the greatest thermal quality, we regularly recorded temperatures exceeding the upper range of the animals’ thermal preference. -

Preliminary Assessment of the Frog Assemblages from Sites Adjacent to Three National Parks in Gabon
Herpetological Conservation and Biology 13(1):240–256. Submitted: 8 August 2017; Accepted: 1 March 2018; Published 30 April 2018. PRELIMINARY ASSESSMENT OF THE FROG ASSEMBLAGES FROM SITES ADJACENT TO THREE NATIONAL PARKS IN GABON JOANNA G. LARSON1,2,3 AND BREDA M. ZIMKUS2 1Department of Ecology and Evolutionary Biology and Museum of Zoology, University of Michigan, Ann Arbor, Michigan 48109, USA 2Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 02138, USA 3Corresponding author, e-mail: [email protected] Abstract.—We report on preliminary frog inventories from three sites in Gabon that are located in close proximity to three national parks. In the lowland forest surrounding Ossélé Village, located north of Batéké Plateau National Park, we documented 14 species from nine genera and six families. The species assemblage within the area of Ossélé Village was markedly different from what is known in the Batéké Plateau National Park from recent inventory work, likely due to the secondary rainforest located outside of the national park and the savanna grassland interspersed with gallery forest within the protected area. We recorded 10 species from eight genera and five families in the buffer zone of Birougou National Park. From the buffer zone of Minkébé National Park, we documented 16 species from eight genera and six families. The majority of these species are widely distributed in the lowland forests of Central Africa. No amphibian surveys have yet been undertaken within Birougou and Minkébe National Parks, but the information provided by these inventories provides insight into species that are likely present since the habitat in these buffer zones is found in the adjacent protected areas. -

The Rufford Foundation Final Report
The Rufford Foundation Final Report Congratulations on the completion of your project that was supported by The Rufford Foundation. We ask all grant recipients to complete a Final Report Form that helps us to gauge the success of our grant giving. The Final Report must be sent in word format and not PDF format or any other format. We understand that projects often do not follow the predicted course but knowledge of your experiences is valuable to us and others who may be undertaking similar work. Please be as honest as you can in answering the questions – remember that negative experiences are just as valuable as positive ones if they help others to learn from them. Please complete the form in English and be as clear and concise as you can. Please note that the information may be edited for clarity. We will ask for further information if required. If you have any other materials produced by the project, particularly a few relevant photographs, please send these to us separately. Please submit your final report to [email protected]. Thank you for your help. Josh Cole, Grants Director Grant Recipient Details Your name TCHASSEM FOKOUA Arnaud Marius Influence of anthropogenic activities on the Project title distribution and conservation status of Amphibians of the Bamenda highlands. RSG reference 19327-2 Reporting period February 2016 - March 2017 Amount of grant £5000 Your email address [email protected] Date of this report March 27th 2017 1. Please indicate the level of achievement of the project’s original objectives and include any relevant comments on factors affecting this. -
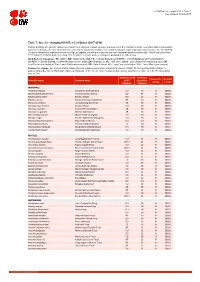
Table 7: Species Changing IUCN Red List Status (2017-2018)
IUCN Red List version 2018-1: Table 7 Last Updated: 05 July 2018 Table 7: Species changing IUCN Red List Status (2017-2018) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2017 (IUCN Red List version 2017-3) and 2018 (IUCN Red List version 2018-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2017) List (2018) change version Category -

Amphibians and National Parks in Gabon, Western Central Africa Amphibien Und Nationalparks in Gabun, Westliches Zentralafrika
©Österreichische Gesellschaft für Herpetologie e.V., Wien, Austria, download unter www.biologiezentrum.at HERPETOZOA 19 (3/4): 135 - 148 135 Wien, 30. Jänner 2007 Amphibians and National Parks in Gabon, western Central Africa Amphibien und Nationalparks in Gabun, westliches Zentralafrika OLIVIER S. G. PAUWELS & MARK-OLIVER RODEL KURZFASSUNG Wir geben eine Zusammenfassung des derzeitigen Wissens über die Amphibieninventare der in Gabun in jüngster Zeit eingerichteten Nationalparks. Vorläufige Arteninventare sind nur für fünf der 13 Parks vorhanden: Crystal, Ivindo, Loango, Lopé und Moukalaba-Doudou. Sechsundsiebzig (86%) der 88 aus Gabun bekannten Arten, alle 10 (100%) der fast endemischen, und drei der sechs (50%) für Gabun endemischen Arten sind aus diesen Nationalparks nachgewiesen. Vorrangig sollte es sein, die Verbreitung der Amphibien in Gabun durch intensive Surveyarbeit besser zu verstehen und neue Schutzgebiete für die Arten zu schaffen, deren Vorkommen noch nicht ausreichend durch die bestehenden Nationalparks abgedeckt sind. ABSTRACT A synthesis of the current state-of-knowledge of amphibian diversity in Gabon and in the recently created Gabonese National Parks is provided. Preliminary inventories are available for five of the 13 parks: Crystal, Ivindo, Loango, Lopé and Moukalaba-Doudou. Seventy-six (86%) of the 88 species known to occur in Gabon, all ten near-endemics (100%), and three of the six Gabonese endemic species (50%) are currently represented in these parks. Future priority actions should comprise an intensified survey -

Journal of Threatened Taxa
ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) Journal of Threatened Taxa 26 January 2019 (Online & Print) Vol. 11 | No. 1 | 13047–13194 PLATINUM 10.11609/jott.2019.11.1.13047-13194 OPEN www.threatenedtaxa.org ACCESS J Building TTevidence for conservation globally ISSN 0974-7907 (Online); ISSN 0974-7893 (Print) Publisher Host Wildlife Information Liaison Development Society Zoo Outreach Organization www.wild.zooreach.org www.zooreach.org No. 12, Thiruvannamalai Nagar, Saravanampatti - Kalapatti Road, Saravanampatti, Coimbatore, Tamil Nadu 641035, India Ph: +91 9385339863 | www.threatenedtaxa.org Email: [email protected] EDITORS Colin Groves, Australian National University, Canberra, Australia Founder & Chief Editor Crawford Prentice, Nature Management Services, Jalan, Malaysia Dr. Sanjay Molur, Coimbatore, India C.T. Achuthankutty, Scientist-G etd.),(R CSIR-National Institute of Oceanography, Goa Dan Challender, University of Kent, Canterbury, UK Managing Editor D.B. Bastawade, Maharashtra, India Mr. B. Ravichandran, Coimbatore, India D.J. Bhat, Retd. Professor, Goa University, Goa, India Dale R. Calder, Royal Ontaro Museum, Toronto, Ontario, Canada Associate Editors Daniel Brito, Federal University of Goiás, Goiânia, Brazil Dr. B.A. Daniel, Coimbatore, India David Mallon, Manchester Metropolitan University, Derbyshire, UK Dr. Ulrike Streicher, Wildlife Veterinarian, Danang, Vietnam David Olson, Zoological Society of London, UK Ms. Priyanka Iyer, Coimbatore, India Davor Zanella, University of Zagreb, Zagreb, Croatia Deepak Apte, Bombay Natural Hisotry Society, Mumbai, India Editorial Advisors Diana Doan-Crider, Texas A&M University, Texas, USA Ms. Sally Walker, Coimbatore, India Dietmar Zinner, German Primate Center, Göttingen, Germany Dr. Robert C. Lacy, Minnesota, USA Dunston P.Ambrose, St. Xavier’s College, Palayamkottai, India Dr.