Actemra® (Tocilizumab) Injection for Intravenous Infusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accuracy of Ontario Health Administrative Databases in Identifying Patients with Rheumatoid Arthritis
ACCURACY OF ONTARIO HEALTH ADMINISTRATIVE DATABASES IN IDENTIFYING PATIENTS WITH RHEUMATOID ARTHRITIS by Jessica Widdifield A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy in Health Services Research Institute of Health Policy, Management & Evaluation University of Toronto © Copyright by Jessica Widdifield 2013 Accuracy of Ontario Health Administrative Databases in Identifying Patients with Rheumatoid Arthritis (RA): Creation of the Ontario RA administrative Database (ORAD) Jessica Widdifield Doctor of Philosophy Institute of Health Policy Management and Evaluation University of Toronto 2013 Abstract Rheumatoid arthritis (RA) is a chronic, destructive, inflammatory arthritis that places significant burden on the individual and society. This thesis represents the most comprehensive effort to date to determine the accuracy of administrative data for detecting RA patients; and describes the development and validation of an administrative data algorithm to establish a province-wide RA database. Beginning with a systematic review to guide the conduct of this research, two independent, multicentre, retrospective chart abstraction studies were performed amongst two random samples of patients from rheumatology and primary care family physician practices, respectively. While a diagnosis by a rheumatologist remains the gold standard for establishing a RA diagnosis, the high prevalence of RA in rheumatology clinics can falsely elevate positive predictive values. It was therefore important we also perform a validation study in a primary care setting where prevalence of RA would more closely approximate that observed in the general population. The algorithm of [1 hospitalization RA code] OR [3 physician RA diagnosis codes (claims) with !1 by a specialist in a 2 year period)] demonstrated a ii high degree of accuracy in terms of minimizing both the number of false positives (moderately good PPV; 78%) and true negatives (high specificity: 100%). -

Rheumatoid Nodules Injected Area Six Times Daily; Nodules Differential Diagnosis and Immunological Appeared in 37 of 82 of Their Patients (450/O)
Matters arising 83 3 Paolaggi J B, Chaouat D, Auquier L. An rheumatic fever, though arthritis or arthralgia but my efforts to reproduce them additional test for the diagnosis and is of a more prolonged nature appearing early experimentally failed miserably! monitoring of giant cell arteritis and after infection. polymyalgia rheumatica. Arth Rheum 1985; F DUDLEY HART Ann Rheum Dis: first published as 10.1136/ard.53.1.83 on 1 January 1994. Downloaded from 28: 837-8. Massell et al' reported in 1937 that nodules 24 Harnnont House, closely resembling those of rheumatic fever 20 Harley Street, could be produced by injecting 3 cc of a London WIN IAN, patient's blood subcutaneously into the soft United Kingdom tissue of an elbow followed by rubbing the 1 Veys E M, De Keyser F. Rheuamtoid nodules: Rheumatoid nodules injected area six times daily; nodules differential diagnosis and immunological appeared in 37 of 82 of their patients (450/o). findings. Ann Rheum Dis 1993; 52: 625-6. 2 Schlesinger B E. The public health aspects of The recent Leader on rheumatoid nodules by I was unable to confirm these findings5 in 40 heart disease in children. Lancet 1938; 1: Veys and De Keyser' gave a very interesting children with rheumatic fever in acute or 593-9, 649-54. and useful review of the subject. When I was convalescent stages, five children with 3 Fink C W. The role of the streptococcus in post- registrar with Dr chronic juvenile arthritis (Still's disease), and streptococcal reactive arthritis in childhood working as a medical polyarteritis nodosa. -

EULAR Points to Consider in the Development of Classification and Diagnostic Criteria in Systemic Vasculitis
EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis Neil Basu1, Richard Watts2, Ingeborg Bajema3, Bo Baslund4, Thorsten Bley5, Maarten Boers6, Paul Brogan7, Len Calabrese8, Maria C. Cid9, Jan Willem Cohen- Tervaert10, Luis Felipe Flores-Suarez11, Shouichi Fujimoto12, Kirsten de Groot13, Loic Guillevin14, Gulen Hatemi15, Thomas Hauser16, David Jayne17, Charles Jennette18, Cees G. M. Kallenberg19, Shigeto Kobayashi20, Mark A. Little21, Alfred Mahr14, John McLaren22, Peter A. Merkel23, Seza Ozen24, Xavier Puechal25, Niels Rasmussen4, Alan Salama26, Carlo Salvarani27, Caroline Savage21, David G. I. Scott28, Mårten Segelmark29, Ulrich Specks30,Cord Sunderkotter31, Kazuo Suzuki32, Vladimir Tesar33, Allan Wiik34, Hasan Yazici15, Raashid Luqmani35 Affiliations 1. University of Aberdeen, Aberdeen, UK 2. University of East Anglia, School of Medicine, Norwich, UK 3. Leiden University Medical Center, Leiden, Netherlands 4. Rigshospitalet, Copenhagen, Denmark 5. University Hospital Freiburg, Freiburg, Germany 6. VU University Medical Center, Amsterdam, Netherlands 7. Institute of Child Health, London, UK 8. Cleveland Clinic Foundation,Cleveland, USA 9. Hospital Clinic, University of Barcelona.IDIBAPS Barcelona, Spain 10. Maastricht UMC, Masstricht, Netherlands 11. Instituto Nacional de Ciencias Médicas y Nutrición, Mexico City, Mexico 12. Miyazaki University, Miyazaki, Japan 13. Klinikum Offenbach, Offenbach, Germany 14. University of Paris Descartes, Paris, France 15. University of Istanbul, Istanbul, Turkey 16. Immunologie-Zentrum Zürich, Zurich, Switzerland 17. Addenbrooke’s Hospital, Cambridge, UK 18. University of North Carolina, Chapel Hill, USA 19. University Hospital Groningen, Groningen, Netherlands 20. Juntendo Koshigaya Hospital, Saitama, Japan 21. Renal Institute of Birmingham , University of Birmingham, Birmingham, UK 22. Whytemans Brae Hospital, Kirkcaldy, UK 23. Boston University School of Medicine, Boston, USA 24. -

Rheumatoid Nodules Developing Under Methotrexate Treatment for Rheumatoid Arthritis
Letters to the Editor 397 Rheumatoid Nodules Developing under Methotrexate Treatment for Rheumatoid Arthritis Sir, several punched-out radiolucencies and non-calcifying nodules Rheumatoid nodules have been reported to occur in about projecting in the soft parts surrounding the joints. 20% of patients with chronic rheumatoid polyarthritis (1, 2). In the recent literature, a correlation between treatment of rheumatoid arthritis with methotrexate and the development DISCUSSION or worsening of rheumatoid nodules has been suggested (2 ± 4). Methotrexate, a common drug for the treatment of rheuma- We describe here the explosive deterioration of rheumatoid toid arthritis, has been used successfully, but various side- nodules during the course of methotrexate treatment for effects have been reported. Among patients with rheumatoid rheumatoid polyarthritis in a 63-year-old woman and discuss arthritis under methotrexate therapy, the ones whose joint current aspects of possible pathogenetic mechanisms as well symptoms are improving due to administration of metho- as therapeutic options for methotrexate-induced rheumatoid trexate frequently develop subcutaneous nodules increasing in nodules. size and number (1 ± 3, 5). Nevertheless, only in rare cases the severity or the localization of the nodules, e.g. vascular, pulmonary or cardiac manifestation, necessitated discontinua- CASE REPORT tion of the drug (3). Cause and effect relationship between methotrexate treatment and the accelerated development of A 63-year-old woman presented to our department with a 15-year nodules is supported by the regression of the nodules after history of chronic rheumatoid polyarthritis affecting her ®ngers, discontinuation of the drug (2, 6). Although the predominant hand, elbow and knee joints. Ten years previously, she ®rst developed a few ®rm nodules on her ®ngers and feet. -

Physiotherapy Co-Management of Rheumatoid Arthritis: Identification of Red flags, Significance to Clinical Practice and Management Pathways
Manual Therapy 18 (2013) 583e587 Contents lists available at SciVerse ScienceDirect Manual Therapy journal homepage: www.elsevier.com/math Professional issue Physiotherapy co-management of rheumatoid arthritis: Identification of red flags, significance to clinical practice and management pathways Andrew M. Briggs a,*, Robyn E. Fary a,b, Helen Slater a,b, Sonia Ranelli a,b, Madelynn Chan c a Curtin Health Innovation Research Institute (CHIRI), Curtin University, GPO Box U 1987, Perth, WA 6845, Australia b School of Physiotherapy, Curtin University, Australia c Department of Rheumatology, Royal Perth Hospital, Australia article info abstract Article history: Rheumatoid arthritis (RA) is a chronic, systemic, autoimmune disease. Physiotherapy interventions for Received 7 December 2012 people with RA are predominantly targeted at ameliorating disability resulting from articular and peri- Received in revised form articular manifestations of the disease and providing advice and education to improve functional ca- 17 January 2013 pacity and quality of life. To ensure safe and effective care, it is critical that physiotherapists are able to Accepted 19 January 2013 identify potentially serious articular and peri-articular manifestations of RA, such as instability of the cervical spine. Additionally, as primary contact professionals, it is essential that physiotherapists are Keywords: aware of the potentially serious extra-articular manifestations of RA. This paper provides an overview of Rheumatoid arthritis Red flags the practice-relevant manifestations -

Complicated Rheumatoid Nodules in Lung
Hindawi Case Reports in Rheumatology Volume 2020, Article ID 6627244, 3 pages https://doi.org/10.1155/2020/6627244 Case Report Complicated Rheumatoid Nodules in Lung Geetha Wickrematilake Sirimavo Bandaranayake Specialized Childrens Hospital, Kandy, Sri Lanka Correspondence should be addressed to Geetha Wickrematilake; [email protected] Received 18 October 2020; Revised 20 November 2020; Accepted 24 November 2020; Published 3 December 2020 Academic Editor: Gregory J. Tsay Copyright © 2020 Geetha Wickrematilake. ,is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A 65-year-old nonsmoker lady carrying a diagnosis of seropositive erosive rheumatoid arthritis for nine years presented with acute shortness of breath, following a spontaneous pneumothorax while on combination therapy with methotrexate, leflunomide, and tocilizumab. Imaging studies revealed multiple cavitory lung nodules, and a transbronchial lung biopsy favoured a diagnosis of rheumatoid lung nodules. Her initial pathological samples were negative for any infectious cause. A follow-up computerized tomography scan (CT scan) confirmed enlargement of lung nodules with a positive antibody test for aspergillosis which needed antifungal therapy, and currently, her arthritis is managed well with rituximab therapy, sulfasalazine, and hydroxychloroquine. 1. Introduction ,e oxygen saturation was 98% while breathing room air, but -

Rheumatoid Vasculitis As an Initial Presentation of Rheumatoid Arthritis
European Journal of Case Reports in Internal Medicine Rheumatoid Vasculitis as an Initial Presentation of Rheumatoid Arthritis Sravani Lokineni, Mohamed Amr, Leela Krishna Teja Boppana, Megha Garg Department of Internal Medicine, Rochester Regional Health, Rochester, NY, USA Doi: 10.12890/2021_002561- European Journal of Case Reports in Internal Medicine - © EFIM 2021 Received: 14/04/2021 Accepted: 16/04/2021 Published: 29/04/2021 How to cite this article: Lokineni S, Amr M, Boppana LKT, Garg M. Rheumatoid vasculitis as an initial presentation of rheumatoid arthritis. EJCRIM 2021;8: doi:10.12890/2021_002561. Conflicts of Interests: The authors declare there are no competing interests. This article is licensed under a Commons Attribution Non-Commercial 4.0 License ABSTRACT Rheumatoid vasculitis is a rare, extra-articular manifestation that can be seen in long-standing rheumatoid arthritis. Here we present the case of a 51-year-old man who presented with arthralgias, skin rash, dyspnoea and generalized leg swelling and who was diagnosed with rheumatoid arthritis flare. LEARNING POINTS • Extra-articular manifestations like rheumatoid vasculitis and pericarditis rarely present as initial manifestations of rheumatoid arthritis. • A high index of suspicion is required to make the diagnosis, especially in an adult who presents with multiorgan manifestations, rash, and a high titre of rheumatoid factor and anti-CCP antibody levels. KEYWORDS Rheumatoid vasculitis, rheumatoid arthritis, pericarditis CASE DESCRIPTION A 51-year-old Caucasian man with a history of tobacco use presented to the emergency department with shortness of breath and progressive pain and swelling in his upper and lower extremities for 3 weeks as well as an erythematous rash over his lower abdomen and thighs that had started 1 day previously. -

Rheumatoid Vasculitis – Case Report
r e v b r a s r e u m a t o l . 2 0 1 5;5 5(6):528–530 REVISTA BRASILEIRA DE REUMATOLOGIA www.reumatologia.com.br Relato de caso ଝ Vasculite reumatoide – Relato de caso a,∗ b b Inah Maria Drummond Pecly , Juan Felipe Ocampo , Guillermo Pandales Ramirez , b c Hedi Marinho de Melo Guedes de Oliveira , Claudia Guerra Murad Saud b e Milton dos Reis Arantes a Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil b Santa Casa da Misericórdia do Rio de Janeiro, Rio de Janeiro, RJ, Brasil c Universidade Federal Fluminense (UFF), Niterói, RJ, Brasil informações sobre o artigo r e s u m o Histórico do artigo: A artrite reumatoide (AR) é uma doenc¸a crônica autoimune inflamatória sistêmica e sua Recebido em 23 de junho de 2013 principal manifestac¸ão é a sinovite persistente, que compromete articulac¸ões periféricas Aceito em 18 de julho de 2014 de forma simétrica. Apesar do seu potencial destrutivo, a evoluc¸ão da AR é muito variável. On-line em 22 de outubro de 2014 Alguns pacientes podem ter apenas um processo de curta durac¸ão oligoarticular com lesão mínima, enquanto outros sofrem uma poliartrite progressiva e contínua e evoluem com Palavras-chave: acometimento de outros órgãos e sistemas, como pele, corac¸ão, pulmões, músculos e mais Vasculite raramente vasos sanguíneos, que leva à vasculite reumatoide. O objetivo deste estudo foi Artrite reumatoide descrever um caso de vasculite reumatoide, uma condic¸ão rara e grave. Vasculite reumatoide © 2013 Elsevier Editora Ltda. -

Non-Bacterial Thrombotic Endocarditis in a Patient With
Case Report http://dx.doi.org/10.4070/kcj.2016.46.3.425 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal Non-Bacterial Thrombotic Endocarditis in a Patient with Rheumatoid Arthritis Jung-Hye Choi, MD1, Jeong-Eun Park, MD1, Jang-Young Kim, MD2, and Taeyoung Kang, MD1 1Department of Rheumatology, 2Department of Cardiology, Yonsei University Wonju College of Medicine, Wonju, Korea Rheumatoid arthritis (RA) is frequently associated with various extra-joint complications. Although rare, thromboembolic complications are associated with high morbidity and mortality. We experienced a very rare case of nonbacterial thrombotic endocarditis (NBTE) and subsequent embolic stroke in a patient with RA. A 72-year-old male with a 15-year history of RA suddenly developed neurologic symptoms of vomiting and dizziness. Brain magnetic resonance imaging revealed recently developed multiple cerebellar and cerebral lacunar infarctions. Echocardiography showed a pulsating mitral valve vegetation involving the posterior cusp of the mitral valve leaflet, which was confirmed as NBTE. Immediate anti-coagulation therapy was started. The NBTE lesion disappeared in follow-up echocardiography after 4 weeks of anti-coagulation treatment. (Korean Circ J 2016;46(3):425-428) KEY WORDS: Endocarditis, non-infective; Arthritis, rheumatoid; Mitral valve. Introduction of life. Although RA patients have an increased risk of cardiovascular events such as myocardial infarction, stroke, cardiac death and Nonbacterial thrombotic endocarditis (NBTE), a very rare condition thromboembolism compared with the general population,2)3) there that refers to a spectrum of noninfectious endocarditis of the has been no report of mitral valvular NBTE in a patient with RA. -

SUPPLEMENTARY APPENDIX 4: Search Strategies Syntax Guide For
SUPPLEMENTARY APPENDIX 4: Search Strategies Pubmed, Embase Perioperative Management - PubMed Search Strategy – March 6, 2016 Syntax Guide for PubMed [MH] = Medical Subject Heading, also [TW] = Includes all words and numbers in known as MeSH the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [SH] = a Medical Subject Heading [TIAB] = Includes words in the title and subheading, e.g. drug therapy abstracts [MH:NOEXP] = a command to retrieve the results of the Medical Subject Heading specified, but not narrower Medical Subject Heading terms Boolean Operators OR = retrieves results that include at least AND = retrieves results that include all the one of the search terms search terms NOT = excludes the retrieval of terms from the search Perioperative Management PubMed Search Strategy – March 6, 2016 Search Query #1 ((((ARTHROPLASTY, REPLACEMENT, HIP[MH] OR HIP PROSTHES*[TW] OR HIP REPLACEMENT*[TIAB] OR HIP ARTHROPLAST*[TIAB] OR HIP TOTAL REPLACEMENT*[TIAB] OR FEMORAL HEAD PROSTHES*[TIAB]) OR (ARTHROPLASTY, REPLACEMENT, KNEE[MH] OR KNEE PROSTHES*[TW] OR KNEE REPLACEMENT*[TW] OR KNEE ARTHROPLAST*[TW] OR KNEE TOTAL REPLACEMENT*[TIAB]) OR (ARTHROPLAST*[TW] AND (HIP[TIAB] OR HIPS[TIAB] OR KNEE*[TIAB])) AND (("1980/01/01"[PDAT] : "2016/03/06"[PDAT]) AND ENGLISH[LANG])) NOT (((("ADOLESCENT"[MESH]) OR "CHILD"[MESH]) -

Rheumatism Video Discloses Center Of
Bulletin Board Highlighting the latest news and research in rheumatology Bulletin Board Bulletin Board Cognitive behavioral therapy in the news... Lead story: improves sleep and pain in people Cognitive behavioral therapy improves sleep and pain in people with osteoarthritis with osteoarthritis pg 503 In brief… pg 504 IRF-8 protein thought to be involved Results published in the Journal of Clinical The CBT-I regime were made up of 8 in causing gum disease, osteoporosis Sleep Medicine revealed that a successful weekly 2 hour classes, which were spread and arthritis pg 504 treatment for older patients with osteoar- throughout the calendar year in an academic thritis and comorbid insomnia is behavioral medical center. Sleep and pain were reviewed Number of US veterans to develop therapy for insomnia (CBT-I). through self-report at the beginning of the rheumatoid vasculitis drops Cognitive behavioral therapy is a form of treatment and at the end of one year. significantlypg 505 psychotherapy that emphasizes the impor- Initially sleep latency dropped by 16.9 Rheumatism video discloses center of tant role of thinking in how we feel and minutes and a year after treatment it was inflammation at an early stagepg 505 what we do. Patients with osteo arthritis found to have decreased by 11 minutes. and comorbid insomnia were reported to Wake after sleep onset was initially reduced have responded well to the therapy and by 37 minutes, which also dropped to Vitello believes that CBT-I could be both their immediate and self-reported 19.9 minutes a year after treatment. Pain incorporated into behavioral interventions sleep appeared to without directly address- improved by 9.7 points initially and 4.7 for pain management in osteoarthritis. -
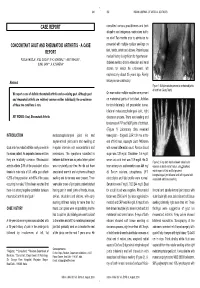
CONCOMITANT GOUT and RHEUMATOID ARTHRITIS - a CASE Presented with Multiple Nodular Swellings on REPORT Feet, Hands, Wrists and Elbows
349 350 INDIAN JOURNAL OF MEDICAL SCIENCES CASE REPORT consulted various practitioners and took allopathic and indigenous medications but to no relief. Two months prior to admission he CONCOMITANT GOUT AND RHEUMATOID ARTHRITIS - A CASE presented with multiple nodular swellings on REPORT feet, hands, wrists and elbows. Patient’s past medical history is significant for hypertension, POOJA KHOSLA*, ATUL GOGIA**, P. K. AGARWAL***, AMIT PAHUJA**, SUNIL JAIN***, K. K. SAXENA# diabetes mellitus, chronic ethanolism and renal stones for which he underwent left nephrectomy about 25 years ago. Family Abstract history is non-contributory. Figure 1: Multiple nodules present on metatarsal joints of both feet (Gouty Tophi) We report a case of definite rheumatoid arthritis and co-existing gout. Although gout On examination multiple nodules were present and rheumatoid arthritis are relatively common entities individually, the co-existence on metatarsal joints of both feet, Achilles of these two conditions is rare. tendon bilaterally, left prepatellar bursa, bilateral metacarpophalangeal joint, right KEY WORDS: Gout, Rheumatoid Arthritis olecranon process. There was swelling and tenderness of PIP and MCP joints of both feet. (Figure 1) Laboratory data revealed INTRODUCTION metatarsophalangeal joint. He had hemoglobin - 8.9gm/dl, ESR 124 mm at the asymmetrical joint pains and swelling at end of first hour, leucocyte count 7400/cmm Gout and rheumatoid arthritis rarely co-exist in irregular intervals with exacerbations and with normal differential count. Random blood the same patient. As separate disease entities remissions. The symptoms subsided in sugar was 139 mg/dl, Creatinine- 1.6 mg/dl; they are relatively common. Rheumatoid between but there was no period when patient serum uric acid level was 10.9 mg/dl.