The FANCM:P.Arg658* Truncating Variant Is Associated with Risk of Triple-Negative Breast Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. Anil Kumar Jha Has Been Appointed As Chairman and Managing Director (CMD) of Coal India
Current Affairs - May 2018 Month All Type All 178 Current Affairs were found in Period - May 11 - 20, 2018 for Type - All Appointments 1. Anil Kumar Jha has been appointed as Chairman and Managing Director (CMD) of Coal India. 2. Badminton Association of India (BAI) President Himanta Biswa Sarma has been elected as vice-president of Badminton Asia Confederation (BAC). 3. Bollywood singer Mohit Chauhan has been named as Green Ambassador of Sikkim by State government. 4. Cabinet Reshuffle - 1. Coal and Railways Minister Piyush Goyal has been given Additional charge of Finance Ministry, until Arun Jaitley is on rest due to health issues. 2. Sports Minister Rajyavardhan Rathore has been given additional charge of Ministry of Information and Broadcasting (Independent Charge). 5. Dilip Tirkey has been appointed as chairman of Odisha Tourism Development Corporation. 6. Former Attoney General Mukul Rohatgi has been appointed as an eminent jurist in selection committee for appointment of Lokpal. 7. Government appointed noted artist and sculptor Uttam Pacharne as regular Chairman of Lalit Kala Akademi. He replaced M.L. Srivastava, who was appointed Protem (Temporary) Chairman recently. 8. Gujarat Governor Om Prakash Kohli has been given additional charge of Madhya Pradesh, while Gujarat Governor Anandiben Patel is on leave. 9. Indian Analyst C Raja Mohan has been appointed as director of Institute of South Asian Studies, a think tank of National University of Singapore. 10. Indian-American Susheela Jayapal has been elected as a member of Board of Commissioners of Multnomah County in Oregon (Western USA State). She is first South Asian to be elected in This western US State. -

Current Affairs May 2018 Appointments 1
Current Affairs May 2018 Appointments 1. Airtel Payments Bank appointed Anubrata Biswas as Managing Director and Chief Executive Officer. 2. Ajay Kumar Mittal has been appointed as acting chief justice of Punjab and Haryana High Court. 3. Alicia Pucheta has been been appointed as South American Nation Paraguay's first woman President (Interim). 4. Amar Devulapalli and Sabina Indrajit have been appointed as President and the Secretary –General of the Indian Journalists Union (IJU) respectively. 5. Anil Kumar Jha has been appointed as Chairman and Managing Director (CMD) of Coal India. 6. Appointments approved by ACC (Appointments Committee of Cabinet) - - Juthika Patankar - as Director General in National Institute of Entrepreneurship and Small Business Development (NIESBUD). - M. Srinivas Rao - Chief Executive Officer in National e-Governance Division (Ministry of Electronics and Information Technology). - Rakesh Kumar Gupta - Secretary in Union Public Service Commission. - Sameer Sharma - Director General in Indian Institute of Corporate Affairs. - Rakesh Kumar Vats - Chairperson in National Pharmaceutical Pricing Authority (NPPA). 7. Badminton Association of India (BAI) President Himanta Biswa Sarma has been elected as vice-president of Badminton Asia Confederation (BAC). 8. Bollywood singer Mohit Chauhan has been named as Green Ambassador of Sikkim by State government. 9. Cab Aggregator Uber promoted its India head Amit Jain as regional general manager for Asia Pacific region (APAC). 10. Cabinet Reshuffle - - Coal and Railways Minister Piyush Goyal has been given Additional charge of Finance Ministry, until Arun Jaitley is on rest due to health issues. - Sports Minister Rajyavardhan Rathore has been given additional charge of Ministry of Information and Broadcasting (Independent Charge). -

January February May June July August
Draw On-Site Minimum Draw On-Site Minimum Draw On-Site Minimum JANUARY M/Q/D Prize Money Ω TFC Ω M/Q/D Prize Money Ω TFC Ω AUGUST M/Q/D Prize Money Ω TFC Ω Brisbane International presented Coupe Rogers présentée par JAN 1 30/32/16 $894,700 $1,000,000 H Porsche Tennis Grand Prix - Stuttgart 28/32/16 $750,900‡ $816,000‡ 56/48/28 - - by Suncorp - Brisbane ^ APR 23 IC AUG 6 Banque Nationale - Montreal H TEB BNP Paribas JAN 1 Shenzhen Open - Shenzhen ^ 32/16/16 $626,750 $750,000 H 32/24/16 $226,750 $250,000 Western & Southern Open - Cincinnati 56/48/28 - - APR 23 Istanbul Cup - Istanbul C AUG 13 H JAN 1 ASB Classic - Auckland ^ 32/32/16 $226,750 $250,000 H APR 30 J&T Banka Prague Open - Prague ^ D! 32/32/16 $226,750 $250,000 C AUG 20 Connecticut Open - New Haven ^ 30/48/16 - - H Grand Prix De SAR La Princesse Sydney International - Sydney ^ D! 30/32/16 $733,900 $799,000 32/32/16 $226,750 $250,000 JAN 8 H APR 30 Lalla Meryem - Rabat ^ D! C AUG 27 US Open - Flushing Meadows* 128/128/64 - - H JAN 8 Hobart International - Hobart ^ 32/24/16 $226,750 $250,000 H MAY SEPTEMBER Coupe Banque MAY 7 Mutua Madrid Open - Madrid ^ 64/32/28 €6,045,855 €6,685,828 C SEP 10 32/24/16 $226,750 $250,000 IH JAN 15 Australian Open - Melbourne*^ 128/96/64 - - H Nationale - Quebec City Internazionali BNL d'Italia - Rome St. -

DJOKOVIC MEETS NADAL in NO. 1 Vs. NO. 2 SHOWDOWN for ROME TITLE
INTERNAZIONALI BNL D’ITALIA: 19 MAY MEDIA NOTES Foro Italico | Rome, Italy | 12-19 May 2019 Draw: S-56, D-32 | Prize Money: €5,207,405 | Surface: Clay ATP Tour Tournament Media ATPTour.com InternazionaliBNLdItalia.com Edward La Cava: [email protected] (ATP PR) Twitter: @ATP_Tour @InteBNLdItalia Angelo Mancuso: [email protected] (Media Desk) Facebook: @ATPTour @InternazionaliBNLdItalia TV & Radio: TennisTV.com DJOKOVIC MEETS NADAL IN NO. 1 vs. NO. 2 SHOWDOWN FOR ROME TITLE • For the second time this season, World No. 1 Novak Djokovic and World No. 2 Rafael Nadal will square off for a prestigious title, as they will take to the court on Sunday to contest the Internazionali BNL d’Italia final. The victor will take the lead in the list of all-time ATP Masters 1000 title winners, as they are currently tied for the most trophies, with 33 ATP Masters 1000 shields apiece. Djokovic leads the head-to-head 28-25, and won their last meeting in straight sets, which was the last time the No. 1- and No. 2-ranked players on the ATP Tour met – this year’s Australian Open final. 8-time champion Nadal and 4-time winner Djokovic have combined to win 12 of the last 14 Rome titles. • Top-ranked Djokovic was pushed to three sets by first-time ATP Masters 1000 semi-finalist Diego Schwartzman in their Saturday encounter, but the Serb prevailed, extending his head-to-head record against Schwartzman to 3-0 and improving to 9-1 in Rome semi-final matches. The 31-year-old has gone 4-4 in his eight previous Rome finals, and seeks to hoist the trophy for the first time since 2015. -

January February May June July August April March
Draw On-Site Minimum Draw On-Site Minimum Draw On-Site Minimum JANUARY M/Q/D Prize Money Ω TFC Ω M/Q/D Prize Money Ω TFC Ω AUGUST M/Q/D Prize Money Ω TFC Ω Rogers Cup presented by DEC 31 Brisbane International - Brisbane ^ 30/32/16 $899,500 $1,000,000 H Porsche Tennis Grand Prix - Stuttgart 28/32/16 -‡ - 56/48/28 - - APR 22 IC AUG 5 National Bank - Toronto H TEB BNP Paribas DEC 31 Shenzhen Open - Shenzhen ^ 32/16/16 $626,750 $750,000 H 32/24/16 $226,750 $250,000 APR 22 Istanbul Cup - Istanbul C AUG 12 Western & Southern Open - Cincinnati 56/48/28 - - H DEC 31 ASB Classic - Auckland ^ 32/32/16 $226,750 $250,000 H Connecticut Open 30/48/16 - - APR 29 J&T Banka Prague Open - Prague ^ D! 32/32/16 $226,750 $250,000 C AUG 19 Tennis - New Haven ^ H Sydney International - Sydney ^ D! 30/24/16 $757,900 $823,000 Grand Prix De SAR La Princesse JAN 7 H 32/32/16 $226,750 $250,000 APR 29 Lalla Meryem - Rabat ^ D! C AUG 26 US Open - Flushing Meadows* 128/128/64 - - H JAN 7 Hobart International - Hobart ^ 32/24/16 $226,750 $250,000 H MAY SEPTEMBER Coupe Banque 64/32/28 - - 32/24/16 $226,750 $250,000 JAN 14 Australian Open - Melbourne*^ 128/96/64 - - H MAY 6 Mutua Madrid Open - Madrid ^ C SEP 9 Nationale - Quebec City IH Hana-cupid Japan 32/32/16 $226,750 $250,000 MAY 13 Internazionali BNL d'Italia - Rome 56/32/28 - - C SEP 9 Women's Open - Hiroshima H St. -

'PDF' Download of the Calendar CLICK HERE
Draw On-Site Minimum Draw On-Site Minimum Draw On-Site Minimum JANUARY M/Q/D Prize Money Ω TFC Ω M/Q/D Prize Money Ω TFC Ω AUGUST M/Q/D Prize Money Ω TFC Ω Rogers Cup presented by DEC 31 Brisbane International - Brisbane ^ 30/32/16 $899,500 $1,000,000 H Porsche Tennis Grand Prix - Stuttgart 28/32/16 -‡ - 56/48/28 - - APR 22 IC AUG 5 National Bank - Toronto H TEB BNP Paribas DEC 31 Shenzhen Open - Shenzhen ^ 32/16/16 $626,750 $750,000 H APR 22 32/24/16 $226,750 $250,000 C Istanbul Cup - Istanbul AUG 12 Western & Southern Open - Cincinnati 56/48/28 - - H DEC 31 ASB Classic - Auckland ^ 32/32/16 $226,750 $250,000 H Connecticut Open 30/48/16 - - APR 29 J&T Banka Prague Open - Prague ^ D! 32/32/16 $226,750 $250,000 C AUG 19 Tennis - New Haven ^ H Sydney International - Sydney ^ D! 30/24-TBC/16 $757,900 $823,000 Grand Prix De SAR La Princesse JAN 7 H 32/32/16 $226,750 $250,000 APR 29 Lalla Meryem - Rabat ^ D! C AUG 26 US Open - Flushing Meadows* 128/128/64 - - H JAN 7 Hobart International - Hobart ^ 32/24/16 $226,750 $250,000 H MAY SEPTEMBER Coupe Banque 64/32/28 - - 32/24/16 $226,750 $250,000 JAN 14 Australian Open - Melbourne*^ 128/96/64 - - H MAY 6 Mutua Madrid Open - Madrid ^ C SEP 9 Nationale - Quebec City IH Hana-cupid Japan 32/32/16 $226,750 $250,000 MAY 13 Internazionali BNL d'Italia - Rome 56/32/28 - - C SEP 9 Women's Open - Hiroshima H St. -
2018 ATP Calendar As of 19
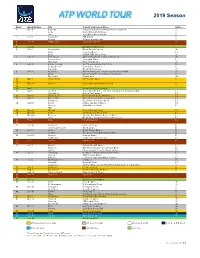
2019 Season Week Week Starting City Current Tournament Name Surface 1 Dec 31 Brisbane Brisbane International presented by Suncorp H Doha Qatar ExxonMobil Open H Pune Tata Open Maharashtra H 2 Jan 7 Auckland ASB Classic H Sydney Sydney International H 3Jan 14 Melbourne Australian Open* H 4 5 Jan 28 Davis Cup First Round* 6 Feb 4 Montpellier Open Sud de France IH Quito Ecuador Open CL Sofia DIEMA XTRA Sofia Open IH 7 Feb 11 Rotterdam ABN AMRO World Tennis Tournament IH Buenos Aires Argentina Open CL New York New York Open IH 8 Feb 18 Rio de Janeiro Rio Open presented by Claro CL Delray Beach Delray Beach Open H Marseille Open 13 Provence IH 9 Feb 25 Acapulco Abierto Mexicano Telcel presentado por HSBC H Dubai Dubai Duty Free Tennis Championships H São Paulo Brasil Open ICL 10 Mar 4 Indian Wells BNP Paribas Open H 11 12 Mar 18 Miami Miami Open presented by Itaú H 13 14 Apr 1 Davis Cup Quarter-finals* 15 Apr 8 Houston Fayez Sarofim & Co. U.S. Men’s Clay Court Championship CL Marrakech Grand Prix Hassan II CL 16 Apr 15 Monte-Carlo Rolex Monte-Carlo Masters CL 17 Apr 22 Barcelona Barcelona Open Banc Sabadell CL Budapest Gazprom Hungarian Open CL 18 Apr 29 Estoril Millennium Estoril Open CL Munich BMW Open by FWU CL TBD 19 May 6 Madrid Mutua Madrid Open CL 20 May 13 Rome Internazionali BNL d’Italia CL 21 May 20 Geneva Banque Eric Sturdza Geneva Open CL Lyon Open Parc Auvergne-Rhône-Alpes Lyon CL 22May 27 Paris Roland Garros* CL 23 24 Jun 10 Stuttgart MercedesCup G ’s-Hertogenbosch Ricoh Open G 25 Jun 17 Halle Gerry Weber Open G London The Queen’s Club Championships G 26 Jun 24 Antalya Antalya Open G Eastbourne Eastbourne International G 27Jul 1 London Wimbledon* G 28 29 Jul 15 Båstad SkiStar Swedish Open CL Newport Dell Technologies Hall of Fame Open G Umag Plava Laguna Croatia Open Umag CL 30 Jul 22 Hamburg German Tennis Championships 2019 CL Atlanta BB&T Atlanta Open H Gstaad J. -

TENNISTAINMENT from the 4Th UNTIL the 13Th of MAY 2018 MASTER 1000 GRAND SLAM Combinados
2018 MUTUA MADRID OPEN TENNISTAINMENT FROM THE 4th UNTIL THE 13th OF MAY 2018 MASTER 1000 GRAND SLAM Combinados MAJOR TOURNAMENTS MUTUA MADRID OPEN Prize Money: 10.878.700 $ Novak Djokovic Dominic Thiem Rafael Nadal THE BEST RACQUETS IN THE WORLD The best ATP racquets in the world will meet in the Mutua Madrid Open, mandatory test of the calendar that brings together the most important players. Angélique Kerber Garbiñe Muguruza Maria Sharapova THE BEST PLAYERS OF A MANDATORY PREMIER As a combined event, the best WTA tennis players will also be present in the Mutua Madrid Open. 49.577.928 visitors on www.madrid-open.com + de 308.871 “likes” on facebook + de 55.728 followers on twitter + de 36,5k pinners on instagram SOCIAL NETWORK < 29 millions Global Audience < 92h.19’53” broadcast TV worldwide TVE - SPANISH TELEVISION Spanish television issued this year 71 matches spread between her 1st channel and Teledeporte. 260.228 Visitors VISITORS With more than 25,000 visitors per day. THE “CAJA MÁGICA” Architect: Dominique Perrault -Location: 17 hectares in the Park of the Manzanares - Features: technological installation with 3 retractable roofs 3 AREAS TENNISTAINMENT STANDS “TENNIS WORLD” 0 LEVEL The "Tennis world" area has converted itself in the ideal showcase for the different sports brands that can exhibit their products generating a great impact and amplifying their notoriety. STANDS OF SPONSORS 0 LEVEL A zone reserved to the tournament visitors where they can interact with the main sponsors. FOODCORNER The FoodCorner area becomes a large Leisure Centre for all kinds of audiences. 58.328 listeners on Set Ball radio* 199.968 viewers on Set Ball TV* * Oficial data of radio & TV SETBALL CIRCUIT The Setball circuit is the tournament 360º producer, we offer to our followers radio signal and Youtube streaming. -
WTA-TV-Schedule 2018
2018 WTA TV SCHEDULE Brisbane International Shenzhen Open ASB Classic Sydney International presented by Suncorp Shenzhen, China Auckland, New Zealand Sydney, Australia Brisbane, Australia January 1 - 7 January 1 - 7 January 2 - 7 January 8 - 13 Hobart International St. Petersburg Ladies Trophy Taiwan Open Qatar Total Open 2018 Hobart, Australia St. Petersburg, Russia Taipei City, Taiwan Doha, Qatar January 8 - 14 January 30 - February 5 January 30 - February 5 February 13 - 18 Dubai Duty Free Abierto Mexicano TELCEL Alya Malaysian Open BNP Paribas Open Tennis Championships presentado por HSBC Kuala Lumpur, Malaysia Indian Wells, CA, USA Dubai, UAE Acapulco, Mexico February 19 - 25 February 27 - March 4 February 27 - March 5 March 8 - 19 Miami Open presented by Itau Volvo Car Open Abierto GNP Seguros Ladies Open Biel Bienne Miami, FL, USA Charleston, SC, USA Monterrey, Mexico Biel, Switzerland March 21 - April 2 April 3 - 9 April 3 - 9 April 10 - 16 Claro Open Colsanitas Porsche Tennis Grand Prix TEB BNP Paribas Istanbul Cup J&T Banka Prague Open Bogota, Colombia Stuttgart, Germany Istanbul, Turkey Prague, Czech Republic April 10 - 15 April 24 - 30 April 24 - 30 May 1 - 6 Grand Prix Mutua Madrid Open Internazionali BNL d'Italia Internationaux de Strasbourg SAR La Princesse Lalla Meyrem Madrid, Spain Rome, Italy Strasbourg, France Rabat, Morocco May 1 - 6 May 6 - 13 May 15 - 21 May 21 - 27 Ricoh Open The Classic Birmingham Mallorca Open The International Eastbourne 's-Hertogenbosch, Netherlands Birmingham, Great Britain Mallorca, Spain -
2018 WTA Calendar
2018 WTA Calendar 2018 WTA 125K Series 2018 ITF WOMEN'S Circuit Calendar As of July 26th, 2018 Prize Money = $115,000 / Total Financial Commitment = $125,000* As of January 22nd, 2017 As of February 6th, 2018 For the official ITF Women's Circuit Calendar, please see the International Tennis Federation website MD Draw Draw WTA MD WTA QLF WTA ITF MD Week Week of Premier Surface Prize Money Ω Minimum TFC Ω International Surface On-Site Prize Money Minimum TFC WTA 125K M/Q/D Surface Week Date Start Date M/Q/D M/Q/D Jobs Per Wk Jobs Per Wk Jobs Per Wk $100,000 $80,000 $60,000 Jobs Per WK WTA + ITF SUN Brisbane International presented by Suncorp - Brisbane ^ H 30/32/16 $894,700 $1,000,000 Shenzhen Open - Shenzhen ^ H 32/16/16 $626,750 $750,000 1 1-Jan 94 80 174 174 1 1-Jan MON ASB Classic - Auckland ^ H 32/32/16 $226,750 $250,000 2 8-Jan SUN Sydney International - Sydney ^ (Doubles !) H 30/32/16 $733,900 $799,000 Hobart International - Hobart ^ H 32/24/16 $226,750 $250,000 62 56 118 118 2 8-Jan 3 15-Jan MON 128 96 224 3 15-Jan Australian Open - Melbourne* ^ 128/96/64 - H 312 4 22-Jan Newport Beach, CA, USA* ($150,000) 32/24/16 H 32 24 56 Andrezieux-Boutheon, FRA 32 4 22-Jan 5 29-Jan MON St. Petersburg Ladies Trophy - St. Petersburg IH 28/32/16 $733,900 $799,000 Taiwan Open - Taipei City, Chinese Taipei IH 32/24/16 $226,750 $250,000 60 56 116 Midland, MI, USA Burnie, AUS 64 180 5 29-Jan 6 5-Feb Fed Cup by BNP Paribas First Round* - TBD 0 6 5-Feb 7 12-Feb MON Qatar Total Open 2018 - Doha (Doubles^) H 56/32/28 $2,872,250 $3,173,000 56 32 88 88 -

Madrid Open 2020 Draw Pdf
Madrid open 2020 draw pdf Continue Men's Singles2019 Mutua Madrid OpenChampion Novak DjokovicRanner-do Stefanos TsitsipasFinal score6-3, 6-4DetailsDraw56 (7 q/4 WC)Seeds16Events Men's Singles women's ← 2018 Mutua Madrid Open 2020 → main article: 2019 Mutua Madrid Open Aleksandr zverev was champion but lost in the quarterfinals to Stefanos Tsitsipazo. Novak Djokovic won the title by beating Tsitsipas in the final, 6-3, 6-4. With the win, Djokovic tied Nadal's record with 33 Masters 1,000 singles titles. It was Roger Federer's first tournament since the 2016 Rome Masters, as well as the last tournament of David Ferrer's professional tennis career. The seeds of the top eight seeds got byes in the second round. 01. Novak Djokovic (champion) 02. Rafael Nadal (Semifinals) 03. Aleksandr Sverev (quarterfinals) 04. Roger Federer (quarterfinals) 05. Dominic Thiem (Semifinals) 06. Kei Nishikori (Third Round) 07. Juan Martin del Potro (Second Round) 08. Stefanos Tsitsipas (Final) 09. Marin Cilic (quarterfinals, withdrawn due to illness) 10. Fabio Fognini (Third Round) 11. Karen Khachanov (Second Round) 12. Daniil Medvedev (First Round) 13. Borna Oric (First Round) 14. Nikoloz Basilashvili (first round) 15. Gael Monfils (Third Round) 16. Marco Cecchinato (First Round) Click on the player's seed number to go to their draw section. Draw Key - Qualifying WC - Wild Card LL - Lucky Loser Alt - Alternative SE - Special Released PR - Protected ITF Ranking - ITF Entry JE - Junior Exempt w/o - Walkover r - Retired d - Default Finals Finals 1 Novak Djokovic -

Genomic Risk Score Impact on Susceptibility to Systemic Sclerosis
Systemic sclerosis Ann Rheum Dis: first published as 10.1136/annrheumdis-2020-218558 on 1 October 2020. Downloaded from TRANSLATIONAL SCIENCE Genomic Risk Score impact on susceptibility to systemic sclerosis Lara Bossini- Castillo,1 Gonzalo Villanueva- Martin ,2 Martin Kerick,2 Marialbert Acosta- Herrera,2 Elena López- Isac,2 Carmen P Simeón,3 Norberto Ortego- Centeno,4 Shervin Assassi ,5 International SSc Group, Australian Scleroderma Interest Group (ASIG), PRECISESADS Clinical Consortium, PRECISESADS Flow Cytometry study group, Nicolas Hunzelmann,6 Armando Gabrielli,7 J K de Vries- Bouwstra,8 Yannick Allanore ,9 Carmen Fonseca,10 Handling editor Josef S 10 11 Smolen Christopher P Denton , Timothy RDJ Radstake, 12 13 5 ► Additional material is Marta Eugenia Alarcón- Riquelme, Lorenzo Beretta , Maureen D Mayes, published online only. To view Javier Martin2 please visit the journal online (http:// dx. doi. org/ 10. 1136/ annrheumdis- 2020- 218558). ABSTRACT Key messages For numbered affiliations see Objectives Genomic Risk Scores (GRS) successfully demonstrated the ability of genetics to identify those end of article. What is already known about this subject? individuals at high risk for complex traits including immune- ► Systemic sclerosis (SSc) is a complex immune- Correspondence to mediated inflammatory diseases (IMIDs). We aimed to mediated disease (IMID) for which a Genomic Dr Lara Bossini- Castillo, test the performance of GRS in the prediction of risk for Risk Score (GRS) has never been implemented. Departamento de Genética e systemic sclerosis (SSc) for the first time. Instituto de Biotecnología, Methods Allelic effects were obtained from the largest Universidad de Granada, Centro What does this study add? de Investigación Biomédica SSc Genome- Wide Association Study (GWAS) to date A SSc GRS discriminates between patients with (CIBM), Parque Tecnológico (9 095 SSc and 17 584 healthy controls with European ► SSc and healthy controls with a remarkable Ciencias de la Salud, Avenida ancestry).