Effect of Fishing Effort on the Catchability of Largemouth Bass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endangered Status for the Ozark Hellbender Salamander
61956 Federal Register / Vol. 76, No. 194 / Thursday, October 6, 2011 / Rules and Regulations Sandra K. Knight, (Braille, large print, electronic files, tariffs electronically over the Internet, Deputy Associate Administrator for audio format), send an e-mail to using the Electronic Tariff Filing System Mitigation, Department of Homeland [email protected] or call the Consumer (ETFS). Additionally, the Commission Security, Federal Emergency Management and Governmental Affairs Bureau at clarifies and makes more consistent Agency. (202) 418–0530 (voice), (202) 418–0432 certain technical rules related to tariff [FR Doc. 2011–25871 Filed 10–5–11; 8:45 am] (TTY). filings. The Commission concludes that BILLING CODE 9110–12–P it is appropriate to apply the same Synopsis electronic filing requirements to all As required by the Paperwork tariff filers and expands the FEDERAL COMMUNICATIONS Reduction Act of 1995 (44 U.S.C. 3507), applicability of the Commission’s rules COMMISSION the FCC is notifying the public that it to include all tariff filers. The received OMB approval on July 20, Commission also concludes that the 47 CFR Parts 61 and 64 2011, for the information collection Commission’s rules, which require [WC Docket No. 10–141; FCC 11–92] requirements contained in the specific formatting and composition of modifications to the Commission’s rules tariffs, will now apply to all tariff filers. Electronic Tariff Filing System (ETFS) in 47 CFR parts 61 and 64. The Chief of the Wireline Competition Under 5 CFR part 1320, an agency Bureau will be responsible for AGENCY: Federal Communications may not conduct or sponsor a collection administering the adoption of electronic Commission. -

Techniques for Catching Chinook Salmon
® TECH KWIKFISH FO R CHINOOK SHEET TECHNIQUES FOR CATCHING CHINOOK SALMON What started over 30 years ago as a local favorite, wrapping a sardine fillet to the belly of large size Kwikfish®, is now the most widely used chinook lure on the West Coast. Kwikfish lures feature high action at all trolling speeds, while their body shape, weight, and lip design make them dive when pulled through the water. Kwikfish can be plunked, trolled or backtrolled with or without weight or in combination with a Jet Diver™. Kwikfish are available in several models ranging in length from 1-3/4 inches for trout up to 5-9/16 inches for salmon. BACKTROLLING With your boat headed upstream above the area you wish to fish, row or run your motor just fast enough to hold your craft even with the current. While holding your boat steady (as if you were anchored), let your diving plug out behind the boat. When you stop letting out line, the current will cause the lure to dive and wiggle. Backtrolling is the process of allowing your boat and trailing lure to slowly slip downstream while resisting the current enough to cause the lure to dive. Knowing that salmon will primarily be found near the bottom, water depth will determine whether you “flatline” your Kwikfish (without a diver or additional weight), or incorporate a Jet Diver or weighted ball as discussed below. FLATLINING The K14, K15 and K16 sized Kwikfish will dive from 8- to 12-feet when trolled or backtrolled on a flat line (without additional weight). -

Master of Puppets
MASTER OF PUPPETS Can you tell a jerkbait from a crankbait, or a swimbait from a wakebait? Knowing how di erent lures work will help you target the right species. Words & Images: Kosta Linardos A Tricky Market to communicating new products on the todiscountaproduct.Wilsonmayjustbea market and providing in-depth reviews, we small Australian company when compared Pretty colours, highly detailed shapes, cool can’t cover every lure. Word of mouth and with American and Japanese tackle giants, packets, effective marketing campaigns and social media play a huge part these days, but they make some amazing products. Some hype can have a more dramatic effect on lure and unfortunately the new and somewhat good anglers could clearly see these lures sales than its actual performance. narcissistic quest for sponsorship and social offered something and had success, and now media notoriety is bringing about some very this cheap crankbait is highly sought-after. Ontheipside,alackofprettycolours, questionable posts and images. The educated highly detailed shapes, cool packets and an angler might have trouble believing certain The same goes for many of the lures from effective marketing campaign can also be shwerecaughtoncertain lures. KajBusch’sBushyLuresbrand;theyare the downfall of a lure that’s actually highly discontinued and in demand, fetching very effectiveintheeld–that’sjustbasicbusiness So, as anglers you need to have a keen high prices online. and marketing. However, as anglers who want understanding of lure type, their intended to be buying the best product that catches us use, how they perform, and when and how It’s a shame these lures didn’t get the shconsistently,weneedtowiseup. -

2020-2021 Regulations Book of Game, Fish, Furbearers, and Other Wildlife
ALABAMA REGULATIONS 2020-2021 GAME, FISH, FURBEARERS, AND OTHER WILDLIFE REGULATIONS RELATING TO GAME, FISH, FURBEARERS AND OTHER WILDLIFE KAY IVEY Governor CHRISTOPHER M. BLANKENSHIP Commissioner EDWARD F. POOLOS Deputy Commissioner CHUCK SYKES Director FRED R. HARDERS Assistant Director The Department of Conservation and Natural Resources does not discriminate on the basis of race, color, religion, age, sex, national origin, disability, pregnancy, genetic information or veteran status in its hiring or employment practices nor in admission to, access to, or operations of its programs, services or activities. This publication is available in alternative formats upon request. O.E.O. U.S. Department of the Interior Washington, D.C. 20204 TABLE OF CONTENTS Division of Wildlife and Freshwater Fisheries Personnel: • Administrative Office .......................................... 1 • Aquatic Education ................................................ 9 • Carbon Hill Fish Hatchery ................................... 8 • Eastaboga Fish Hatchery ...................................... 8 • Federal Game Agents ............................................ 6 • Fisheries Section ................................................... 7 • Fisheries Development ......................................... 9 • Hunter Education .................................................. 5 • Law Enforcement Section ..................................... 2 • Marion Fish Hatchery ........................................... 8 • Mussel Management ............................................ -

Annual Report 2009 CONTENT
Annual Report 2009 CONTENT Business Operations and Strategy • Crafted from Experience – Positioned for Further Growth …………………… 4-5 • Rapala and Year 2009 in Brief .…………………… 6-7 • Statement by President and CEO ………………… 8-9 • Strategy, Strengths and Priorities ……………… 10-11 • Group Fishing Products – Lures ……………… 12-15 • Group Fishing Products – Hooks ……………… 16-17 • Group Fishing Products – Lines ……………… 18-19 • Group Fishing Products – Accessories ……… 20-21 • Other Group Products ………………………… 22-23 • Third Party Products – Fishing ………………… 24-25 • Third Party Products – Hunting, Winter Sports and Outdoor …………………… 26-27 • World Champions ………………………………… 28 Financial Statements • Review of the Board of Directors ……………… 30-32 • Auditors’ Report …………………………………… 33 • Consolidated Financial Statements, IFRS ……… 34-67 • Key Financial Figures …………………………… 68-70 • Parent Company Financials, FAS ……………… 71-72 • Risk Management ……………………………… 73-75 • Corporate Responsibility and Sustainable Development ……………………… 76-80 • Shares and Shareholders ……………………… 81-83 • Board of Directors and Management …………… 84-85 Investor Information • Corporate Governance Statement ……………… 86-88 • Shareholder Information …………………………… 89 • Locations of Business Operations ……………… 90-91 • Examples of Rapala Product Range …………… 92-93 RAPALA ANNUAL REPORT 2009 22 Rapala VMC Corporation (Rapala) is a leading fishing tackle company and the global market leader in fishing lures, treble hooks and fishing related knives and tools. The Group also has a strong global position in other fishing catego- ries and it is one of the leading distributors of outdoor, hunting and winter sport products in the Nordic countries. The Group has the largest distribution network in the industry. The main manufacturing facilities are located in Finland, France, Estonia, Russia and China. The Group brand portfolio includes the leading brand in the industry, Rapala, and other global brands like VMC, Storm, Blue Fox, Luhr Jensen, Wil- liamson, Marttiini and Sufix. -

Guide to the Classification of Fishing Gear in the Philippines
U.S. Department of Agriculture Animal and Plant Health Inspection Service Wildlife Services Historic document – Content may not reflect current scientific research, policies or practices. GUIDE TO THE CLASSIFICATION OF FISHING GEAR IN THE PHILIPPINES By AGUSTIN F. UMALI, Ichthyologist Illustrations by Silas G. Duran RESEARCH REPORT 17 Fish and Wildlife Service, Albert M. Day, Director United States Department of the Interior, Oscar L. Chapman, Secretary UNITED STATES GOVERNMENT PRINTING OFFICE : 1950 For sale by the Superintendent of Documents, United States Government Printing Office Washington 25, D. C. - Price 40 cents ABSTRACT One of the serious handicaps in the administration and manage ment of the fisheries of the Philippines has been the lack of standardized nomenclature for fishing gear. This publication attempts to solve the problem. It is divided into five parts: The first presents a basic clas sification of fishing gear; the second is a key by means of which fishing gear can be identified and new terms for fishing gear can be properly classified; the third defines and illustrates various types of fishing gear; the fourth is a tabular classification of local Filipino di alect names; and the fifth is a glossary including definitions of more than a thousand terms. CONTENTS Classification of the Gear. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 2 Part I. Basic Classification of Fishing Gear. • • • • • • • . • • • • • • • • • 6 Part II. Key to the Identification of Classes of Fishing Gear. • . • -

Tips for Catching Small Lake Trout
10-Dec-18 Tips for Catching Small Lake Trout The Wyoming Game and Fish Department and the Utah Division of Wildlife Resources manage Flaming Gorge Reservoir (FGR) to provide fishing opportunities for a variety of anglers, the majority of which visit the reservoir to fish for Kokanee Salmon and Rainbow Trout. Kokanee and Rainbow Trout are not only prized sportfish but also the forage that helps maintain the Trophy Lake Trout population in FGR. Population surveys completed over the last three decades have shown an increase in the abundance of small Lake Trout (e.g., less than 28 inches). If the existing population of small fish is not reduced, there could be too much competition to sustain trophy Lake Trout. Angler harvest is the most viable option for reducing numbers of small Lake Trout. Once Lake Trout obtain a large enough size, they are highly-effective predators that prey heavily on Kokanee and Rainbow Trout. The predator/prey relationship must be managed to maintain balance between all species. In FGR, this relationship is on the verge of imbalance due to the increase in the number of predators, specifically Lake Trout <28 inches. When Lake Trout reach a length of 25-inches, they typically consume fish. If the existing population of small fish (< 28- inches) is not reduced, there will not be enough Kokanee or Rainbow Trout to support the Lake Trout population. Anglers are encouraged to target and harvest Lake Trout <28-inches. Small Lake Trout are delicious and provide substantial fillets when you consider the liberal limit on fish <28-inches. -
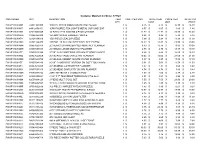
Container Upc Description Item Qty Cost
Container Manifest for Order: 379729 CONTAINER UPC DESCRIPTION ITEM COST PER UNIT TOTAL ITEM PRICE PER TOTAL ITM QTY COST UNIT PRICE FWAP113WAMF 72481925909 1000 PC WHITE MOUNTAIN RETRO PUZZLE 1 $ 6.75 $ 6.75 $ 16.99 $ 16.99 FWAP113WANT 64412456151 10PK POURED TEA LIGHTS METAL CUP UNSCENT 1 $ 0.57 $ 0.57 $ 1.49 $ 1.49 FWAP113WAM3 87519500349 14' RATCHETX 3000LBS 4 PACK ORANGE 1 $ 21.72 $ 21.72 $ 33.89 $ 33.89 FWAP113WDHD 01864365029 15 LOOP SCARF HANGER-PURPLE 1 $ 2.26 $ 2.26 $ 5.99 $ 5.99 FWAP113WAT4 02108134019 1500 PIECE CEACO PUZZLE 1 $ 5.94 $ 5.94 $ 11.99 $ 11.99 FWAP113WAMH 88591144470 15575M - 29 PC HIGH SPEED STL SET 15575M 1 $ 18.83 $ 18.83 $ 29.99 $ 29.99 FWAP113WAMH 03857659378 2018EAAG DAYMINDER POLY MONTHLY PLANNER 2 $ 8.39 $ 16.78 $ 19.99 $ 39.98 FWAP113WAMH 03857657088 2018EAAG LARGE MONTHLY PLANNER 1 $ 2.75 $ 2.75 $ 19.99 $ 19.99 FWAP113WATE 03857626399 2019E-AAG CONTEMPO LRG WM SENZO PLANNER 1 $ 8.44 $ 8.44 $ 16.89 $ 16.89 FWAP113WAM5 03857662669 2019E-AAG PAIGE LARGE WM PLANNER 1 $ 10.02 $ 10.02 $ 21.09 $ 21.09 FWAP113WAMX 03857649759 2019E-AAG SORBET SM WM PAPER PLANNER 1 $ 8.97 $ 8.97 $ 17.99 $ 17.99 FWAP113WATE 03857648289 2019E-CAMBRIDGE ARIZONA SM SOFT TOUCH WM 1 $ 5.01 $ 5.01 $ 11.59 $ 11.59 FWAP113WAT4 03857645089 2019E-MEAD CAPRICE PKT PLANNER 1 $ 1.37 $ 1.37 $ 3.69 $ 3.69 FWAP113WANX 03857633819 2019E-MEAD SIMPLICITY SM WM PLANNER 1 $ 4.74 $ 4.74 $ 9.49 $ 9.49 FWAP113WAMH 78630913742 24PC GIFTBAGS X JUMBO IN PDQ 1 $ 1.60 $ 1.60 $ 4.99 $ 4.99 FWAP113WANT 03885805654 3 1/2" X 7" TEARDROP TERRARIUM 3756-06-0 1 -

Montana Fishing Regulations
MONTANA FISHING REGULATIONS 20March 1, 2018 — F1ebruary 828, 2019 Fly fishing the Missouri River. Photo by Jason Savage For details on how to use these regulations, see page 2 fwp.mt.gov/fishing With your help, we can reduce poaching. MAKE THE CALL: 1-800-TIP-MONT FISH IDENTIFICATION KEY If you don’t know, let it go! CUTTHROAT TROUT are frequently mistaken for Rainbow Trout (see pictures below): 1. Turn the fish over and look under the jaw. Does it have a red or orange stripe? If yes—the fish is a Cutthroat Trout. Carefully release all Cutthroat Trout that cannot be legally harvested (see page 10, releasing fish). BULL TROUT are frequently mistaken for Brook Trout, Lake Trout or Brown Trout (see below): 1. Look for white edges on the front of the lower fins. If yes—it may be a Bull Trout. 2. Check the shape of the tail. Bull Trout have only a slightly forked tail compared to the lake trout’s deeply forked tail. 3. Is the dorsal (top) fin a clear olive color with no black spots or dark wavy lines? If yes—the fish is a Bull Trout. Carefully release Bull Trout (see page 10, releasing fish). MONTANA LAW REQUIRES: n All Bull Trout must be released immediately in Montana unless authorized. See Western District regulations. n Cutthroat Trout must be released immediately in many Montana waters. Check the district standard regulations and exceptions to know where you can harvest Cutthroat Trout. NATIVE FISH Westslope Cutthroat Trout Species of Concern small irregularly shaped black spots, sparse on belly Average Size: 6”–12” cutthroat slash— spots -

Fishing for Fairness Poverty, Morality and Marine Resource Regulation in the Philippines
Fishing for Fairness Poverty, Morality and Marine Resource Regulation in the Philippines Asia-Pacific Environment Monograph 7 Fishing for Fairness Poverty, Morality and Marine Resource Regulation in the Philippines Michael Fabinyi Published by ANU E Press The Australian National University Canberra ACT 0200, Australia Email: [email protected] This title is also available online at: http://epress.anu.edu.au/ National Library of Australia Cataloguing-in-Publication entry Author: Fabinyi, Michael. Title: Fishing for fairness [electronic resource] : poverty, morality and marine resource regulation in the Philippines / Michael Fabinyi. ISBN: 9781921862656 (pbk.) 9781921862663 (ebook) Notes: Includes bibliographical references and index. Subjects: Fishers--Philippines--Attitudes. Working poor--Philippines--Attitudes. Marine resources--Philippines--Management. Dewey Number: 333.91609599 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher. Cover design and layout by ANU E Press Cover image: Fishers plying the waters of the Calamianes Islands, Palawan Province, Philippines, 2009. Printed by Griffin Press This edition © 2012 ANU E Press Contents Foreword . ix Acknowledgements . xiii Selected Tagalog Glossary . xvii Abbreviations . xviii Currency Conversion Rates . xviii 1 . Introduction: Fishing for Fairness . 1 2 . Resource Frontiers: Palawan, the Calamianes Islands and Esperanza . 21 3 . Economic, Class and Status Relations in Esperanza . 53 4 . The ‘Poor Moral Fisher’: Local Conceptions of Environmental Degradation, Fishing and Poverty in Esperanza . 91 5 . Fishing, Dive Tourism and Marine Protected Areas . 121 6 . Fishing in Marine Protected Areas: Resistance, Youth and Masculinity . -

Toolsand Accessories
Tools and aRapala offersccessories a full range of tools and accessories from pliers to scales and landing nets and fillet knives. Some of them are true, trusted classics like the Fish ‘N Fillet knife – world’s most sold fillet knife that has served fishermen for over five decades – while others are on the leading edge of modern technology, innovation and design like the Rapala Custom Design series. Rapala’s proprietary designs have been created and manufactured to meet fishermen’s needs worldwide, from ultralight fishing on lakes and streams to heavy-duty saltwater pursuits. The classic to modern designs reinforce our commitment to style and functional design. 104 Tools and aRapala offersccessories a full range of tools and accessories from pliers to scales and landing nets and fillet knives. Some of them are true, trusted classics like the Fish ‘N Fillet knife – world’s most sold fillet knife that has served fishermen for over five decades – while others are on the leading edge of modern technology, innovation and design like the Rapala Custom Design series. Rapala’s proprietary designs have been created and manufactured to meet fishermen’s needs worldwide, from ultralight fishing on lakes and streams to heavy-duty saltwater pursuits. The classic to modern designs reinforce our commitment to style and functional design. 105 rapala cusTom design series Modern innovations and contemporary design lifts angler’s tools to a totally new level. Features like Rapala’s proprietary Mag Spring have not been seen on any tool before. Clean looks and well thought-out functionality raise the bar on how fisherman’s tools are made. -

Marine Aquaculture and Stock Enhancement in Florida
MARINE AQUACULTURE AND STOCK ENHANCEMENT IN FLORIDA: RESEARCH AND DEVELOPMENT FLORIDA PROJECT, FWC CONTRACT No. 01258, AMENDMENT No.1 MML Project No. 170.355, 170.385, 170.552, 170.553 FINAL REpORT J UNE 1, 2003 THROUGH MAy 9, 2004 PREPARED FOR: MR. B ILL HALSTEAD FLORIDA F ISH AND W ILDLIFE C ONSERVATION COMMISSION STOCK E NHANCEMENT RESEARCH F ACILITY 14495 HARLLEE R OAD ~ PORT MANATEE, FLORIDA 34221 EDITED By: MEAGHAN C. D ARCY, M .S. CONTRIBUTORS: NATHAN BRENNAN, M.S. ~ CAROLE N EIDIG, M .S. KEv AN MAIN, PH.D . ~ KEN LEBER, PH.D. MIKE NYSTROM, M.S. ~ D AVE JENKINS, B .S. STOCK ENHANCEMENT PROGRAM, CENTER FOR FISHERIES ENHANCEME T M OTE MARINE LABORATORY 1600 KEN THOMPSON PARKWAY ~ SARASOTA, FLORIDA 34236 April 19, 2004 Mote Marine Laboratory Technical Report No. 964 This document is printed on recycled paper with non destructive ink 1600 Ken Thompson Parkway Myra Monfort Runyan Chairman of the Board Sa rasota, Flo rida 34236 -1096 USA M&TE PHONE: (941 ) 388-4441 FAX: (941) 388 -4312 Kumar Mahadevan. Ph.D. ViARINE LABORATORY INTERNET: [email protected] • www.mote.org President FIELDST ATIONS: Florida Keys. 24244 Ove rseas Highway · Summerland Key. FL 33042 • PHONE: (305j 745-2729 . FAX: (305) 745-2730 Charlotte Harbor· P.O. Box 2197 · Pineland, FL 33945 · PHONE: (239) 283-i622 • FAX: (239) 283-2466 Mot, AqUJzculture Park • 12300 Fruitville Roa.d • Sarasota, FL 34240 May 7, 2004 Mr. Bill Halstead FLORIDA FISH AND WILDLIFE CONSERVATION COMMISSION Stock Enhancement Research Facility 14495 Harllee Road Port Manatee, Florida 34221 Re: June I, 2003 - May 9, 2004, Final Report Draft: "Marine Aquaculture and Stock Enhancement in Florida: Research and Development." FWC Contract No.