Biological Response to Environmental Flows Below Corin, Bendora, Cotter and Googong Dams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACT Water Quality Report 1997-98
ACT Water Quality Report 1997-98 Environment ACT i ACT Water Quality Report 1997 - 98 Further Information: Raw data for all of the sites reported are available on the Internet under the ACT Government web site at www.act.gov.au/Water_Quality/start.cfm Should you wish to seek further information in relation to this report, please contact: Greg Keen Water Unit Environment ACT Telephone: 6207 2350 Facsimile: 6207 6084 E-mail: [email protected] ii Environment ACT ACT Water Quality Report 1997-98 Contents List of Figures ................................................................................................................................iv List of Tables ..................................................................................................................................iv Executive Summary.........................................................................................................................1 Introduction.....................................................................................................................................2 Purpose .......................................................................................................................................................2 Scope ...........................................................................................................................................................2 Landuse.......................................................................................................................................................2 -

SURVEY of VEGETATION and HABITAT in KEY RIPARIAN ZONES of TRIBUTARIES of the MURRUMBIDGEE RIVER in the ACT: Naas, Gudgenby, Paddys, Cotter and Molonglo Rivers
SURVEY OF VEGETATION AND HABITAT IN KEY RIPARIAN ZONES OF TRIBUTARIES OF THE MURRUMBIDGEE RIVER IN THE ACT: Naas, Gudgenby, Paddys, Cotter and Molonglo Rivers Lesley Peden, Stephen Skinner, Luke Johnston, Kevin Frawley, Felicity Grant and Lisa Evans Technical Report 23 November 2011 Conservation Planning and Research | Policy Division | Environment and Sustainable Development Directorate TECHNICAL REPORT 23 Survey of Vegetation and Habitat in Key Riparian Zones of Tributaries of the Murrumbidgee River in the ACT: Naas, Gudgenby, Paddys, Cotter and Molonglo Rivers Lesley Peden, Stephen Skinner, Luke Johnston, Kevin Frawley, Felicity Grant and Lisa Evans Conservation, Planning and Research Policy Division Environment and Sustainable Development Directorate GPO Box 158, CANBERRA ACT 2601 i Front cover: The Murrumbidgee River and environs near Tharwa Sandwash recreation area, Tharwa, ACT. Photographs: Luke Johnston, Lesley Peden and Mark Jekabsons. ISBN: 978‐0‐9806848‐7‐2 © Environment and Sustainable Development Directorate, Canberra, 2011 Information contained in this publication may be copied or reproduced for study, research, information or educational purposes, subject to appropriate referencing of the source. This document should be cited as: Peden, L., Skinner, S., Johnston, L., Frawley, K., Grant, F., and Evans, L. 2011. Survey of Vegetation and Habitat in Key Riparian Zones in Tributaries of the Murrumbidgee River in the ACT: Cotter, Molonglo, Gudgenby, Naas and Paddys Rivers. Technical Report 23. Environment and Sustainable Development Directorate, Canberra. Published by Conservation Planning and Research, Policy Division, Environment and Sustainable Development Directorate. http://www.environment.act.gov.au | Telephone: Canberra Connect 132 281 ii ACKNOWLEDGEMENTS This document was prepared with funding provided by the Australian Government National Action Plan for Salinity and Water Quality. -

REVIEW of the ACT WATER RESOURCES ENVIRONMENTAL FLOW GUIDELINES 2013 November 2017 Final Report to Environment, Planning and Sustainable Development Directorate
REVIEW OF THE ACT WATER RESOURCES ENVIRONMENTAL FLOW GUIDELINES 2013 November 2017 Final Report to Environment, Planning and Sustainable Development Directorate. APPLIEDECOLOGY.EDU.AU ACT ENVIRONMENTAL FLOW GUIDELINES: REVIEW Prepared for: Environment, Planning and Sustainable Development Directorate, ACT Government Produced by: Institute for Applied Ecology appliedecology.edu.au University of Canberra, ACT 2601 Telephone: (02) 6201 2795 Facsimile: (02) 6201 5651 Authors: Dr. Adrian Dusting, Mr. Ben Broadhurst, Dr. Sue Nichols, Dr. Fiona Dyer This report should be cited as: Dusting,A., Broadhurst, B., Nichols, S. and Dyer, F. (2017) Review of the ACT Water Resources Environmental Flow Guidelines 2013. Final report to EPSDD, ACT Government. Institute for Applied Ecology, University of Canberra, Canberra. Inquiries regarding this document should be addressed to: Dr. Fiona Dyer Institute for Applied Ecology University of Canberra Canberra 2601 Telephone: (02) 6201 2452 Facsimile: (02) 6201 5651 Email: [email protected] Document history and status Version Date Issued Reviewed by Approved by Revision Type Draft 07/08/2017 IAE EFG review Adrian Dusting Internal team Final 11/08/2017 Adrian Dusting Fiona Dyer Internal Final - revised 15/11/2017 ACT Gov. steering Adrian Dusting External committee, EFTAG, MDBA Front cover photo: Cotter River at Top Flats. Photo by Fiona Dyer APPLIEDECOLOGY.EDU.AU ii ACT ENVIRONMENTAL FLOW GUIDELINES: REVIEW TABLE OF CONTENTS Executive Summary ......................................... vii Background and -
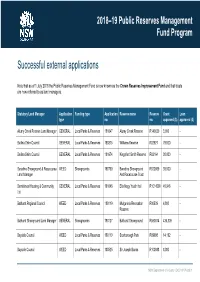
Successful External Applications
2018–19 Public Reserves Management Fund Program Successful external applications Note that as of 1 July 2018 the Public Reserves Management Fund is now known as the Crown Reserves Improvement Fund and that trusts are now referred to as land managers. Statutory Land Manager Application Funding type Application Reserve name Reserve Grant Loan type no. no. approved ($) approved ($) Alumy Creek Reserve Land Manager GENERAL Local Parks & Reserves 181647 Alumy Creek Reserve R140020 3,600 - Ballina Shire Council GENERAL Local Parks & Reserves 180875 Williams Reserve R82927 79,000 - Ballina Shire Council GENERAL Local Parks & Reserves 181674 Kingsford Smith Reserve R82164 30,000 - Baradine Showground & Racecourse WEED Showgrounds 180790 Baradine Showground R520059 38,500 - Land Manager And Racecourse Trust Barriekneal Housing & Community GENERAL Local Parks & Reserves 181646 Ella Nagy Youth Hall R1014508 40,946 - Ltd Bathurst Regional Council WEED Local Parks & Reserves 180119 Mulgunnia Recreation R80539 4,800 - Reserve Bathurst Showground Land Manager GENERAL Showgrounds 180127 Bathurst Showground R590074 435,309 - Bayside Council WEED Local Parks & Reserves 180110 Scarborough Park R69998 14,192 - Bayside Council WEED Local Parks & Reserves 180525 Sir Joseph Banks R100088 8,000 - NSW Department of Industry | DOC18/176333| 1 2018–19 Public Reserves Management Fund Program Statutory Land Manager Application Funding type Application Reserve name Reserve Grant Loan type no. no. approved ($) approved ($) Bayside Council WEED Local Parks & Reserves -

Aboriginal Cultural Heritage Assessment ______
APPENDIX O ABORIGINAL CULTURAL HERITAGE ASSESSMENT _____________________________________________________________________________ Snowy 2.0 Exploratory Works Aboriginal Cultural Heritage Assessment Report Date: 20 July 2018 Author: Dr Julie Dibden NSW Archaeology Pty Ltd Proponent: Snowy Hydro Limited Local Government Area: Snowy Valleys & Snowy Monaro Regional Councils TABLE OF CONTENTS EXECUTIVE SUMMARY........................................................................................... 1 1. INTRODUCTION ................................................................................................... 9 1.1 THE PROJECT ...................................................................................................... 9 1.2 PURPOSE OF THIS REPORT ..................................................................................10 1.3 LOCATION OF EXPLORATORY WORKS .................................................................10 1.4 PROPONENT .......................................................................................................12 1.5 ASSESSMENT GUIDELINES AND REQUIREMENTS ...............................................12 2. DESCRIPTION OF THE PROJECT – EXPLORATORY WORKS .......................14 2.1 THE EXPLORATORY WORKS.................................................................................14 2.2 EXPLORATORY TUNNEL......................................................................................15 2.3 PORTAL CONSTRUCTION COMPOUND .................................................................20 -

Water and Sewerage Services Connection and Supply
Recycled Water Services Connection and Supply Standard Customer Contract Contents 1 Enquiries and complaints Page 2 2 What obligations do you have? Page 2 Commencement of supply Keep us informed of changes Life support equipment Drinking water and sewerage services Obligations in relation to use of recycled water Leasing your premises to others Your equipment Not for use of fire services Bypassing the meter Other obligations affecting the premises Other customer obligations 3 Connection work Page 5 4 What standard of service can you expect? Page 6 Continuous supply, quality and pressure not guaranteed Correction Correction of interruptions to your supply Planned interruptions Unplanned interruptions Drought 5 What other rights do you have? Page 7 Ask for identification Are you having difficulties paying an account? Outstanding water and sewerage charges You may request other information Plumbing fault in our network Undetected leak Work done by us on your premises You may request disconnection 6 Charges Page 9 Schedule of Charges Commencement of charges Extension and equipment costs Other charges ii Payment of accounts Errors in accounts Disputed accounts GST 7 Can you be required to provide a security deposit? Page 11 8 Measuring your water consumption Page 11 Meter reading and inspections Check readings Installation of meters Ownership of meters Estimating your water consumption Testing of meters at your request We may initiate test Faulty meters or incorrect readings Maintenance of meters Protecting -

3F38373009842c86616a
•CANBERRA ' BUSH WALKING •CLUB INC NEWSLETTER GPO Box 160, Canberra ACT 2601 VOLUMB 34 SEPTEI'ffiER 1998 NUMBER 9 ANNUAL GENERAL MEETING Greg BUckley Award & other Prizes 8pm Wednesday 16 September Dickson Library Community Room (entrance at rear of library) Make the most of the evening and Join other members at 6.00pm for a convivial (B'YO) meal at the Pho P1w Quoc Restaurant in Cape Street, Dickson. Try to be early to ensure there will be ample time to finish and still get to the meetIng In comfortable time Walks to Allan Mikkelsen Ph: 6278 3164 E-mail: [email protected] SD Century Courts, 4 Beetaloo St, Hawker ACT 2614 Articles etc. for publication to Paul Edstein Ph: 6271 4514(w) 6288 1398 (h) Fax: 6271 4560 (w) E-mail: [email protected] 19 Gamor St, Waramanga ACT 2611 PRESIDENT'S PRATTLE And so to my final "Prattle". It doesn't seem those people, but also to everyone who has supported like I've been compiling "Prattles" for two years, so I walks, other activities and Club meetings. think I must have enjoyed it! As the saying goes "time flies when you're having fun". I have to admit though I would just like to mention here that activity leaders there were months when I really had to search for do have one important obligation when they something I thought was worth printing - okay, not return home from the activity. That is to phone the everyone thought it was worth printing! Some Check-in Officer (as early as possible or at least members didn't agree with what I had to say on within 24 hours) and advise the safe completion of the occasions and I will always think that was good for trip and the number of people who went - and the Club. -

Tourist Drive 7 Yass to Tumut
A B C D E F G H Source: © Department of Lands TO GRENFELL 24km TO COWRA 27km TO OBERON 44km Panorama Avenue Bathurst 2795 HENRY Koorawatha Wyangala www.lpi.nsw.gov.au Dam © Copyright LPI &CartoscopePtyLtd CARTOSCOPE www.maps.com.au LAWSON River HWY RD 22 1 RD 1 Yass to TumutA41 - stay on the Tourist Drive 7 Bendick Creek WAY Murrell 23 BIGGA 28 14 River 82 29 POINT Distance: From Wee Jasper to TuenaTumut you pass through dry B81 Yass to Wee Jasper 52 km - allow 1hr 14 sclerophyll forest, farming land and pine forests. RD RD River MILVALE Frogmore River Murruin Wee Jasper toYOUNG Tumut 68km - allow 1.15hr Beautiful multi-agedCk stands of eucalypt with an 21 Bicentennial JUNCTION 11 National Trail Chinaman's 13 understory dominated by11 hard leafed shrubs, grasses, Dam 25 WAY Creek Bolong 2 MOPPITY TO TEMORA 43km OLYMPIC 2 Road conditions: bracken and fern, line the roads and go back into the 20 14 23 Tourist Drive 7 Yass to Wee17 A41 Jasper - Sealed forest depths. BINDA 18 R RD 16 RD 10 Wee Jasper to Tumut - Unsealed - not recommendedBOOROWA Wallendbeen 50 TYRL 34 RD 8 TYRL for caravans or long vehicles LAGGAN TARALGA 28 20 RD Taralga 18 HARDEN RD Road conditionsMURRUMBURRAH and travelling times will depend on CROOKWELL 5 TO TEMORA 39km BURLEY CARRABUNGLA 45 theBradman's weather Birthplace and your vehicle.24 RD 3 14 3 COOTAMUNDRA 12 Drive carefully along Tourist Drive 7 as there is often Windfarm RD B94 7 GRIFFIN Pejar Dam stock and wildlife on the road.Gilbert's Grave River GULLEN 42 VALLEY 48 30 Tarlo 14 41 Ck River TO JUNEE 43km 20 -

Characterisation of Flow in Regulated and Unregulated Streams in Eastern Australia
Characterisation of Flow in Regulated and Unregulated Streams in Eastern Australia Characterisation of Flow in Regulated and Unregulated Streams in Eastern Australia Jane Growns Murray–Darling Freshwater Research Centre Nick Marsh Centre for Environmental Applied Hydrology Cooperative Research Centre for Freshwater Ecology Technical Report 3/2000 September 2000 Centre for Environmental Applied Hydrology Cooperative Research Centre for Freshwater Ecology i Characterisation of Flow in Regulated and Unregulated Streams in Eastern Australia The Cooperative Research Centre for Freshwater Ecology improves the health of Australia’s rivers, lakes and wetlands through research, education and knowledge exchange. It was established in 1993 under the Australian Government’s Cooperative Research Centre Program. The Cooperative Research Centre for Freshwater Ecology is a collaborative venture between: • ACTEW Corporation • CSIRO Land and Water • Department of Land and Water Conservation, NSW • Department of Natural Resources, Queensland • Department of Natural Resources and Environment, Victoria • Environment ACT • Environment Protection Authority, NSW • Environment Protection Authority, Victoria • Goulburn–Murray Rural Water Authority • Griffith University • La Trobe University • Lower Murray Water • Melbourne Water • Monash University • Murray-Darling Basin Commission • Murray-Darling Freshwater Research Centre • Sunraysia Rural Water Authority • Sydney Catchment Authority • University of Canberra Cooperative Research Centre for Freshwater Ecology Ph: 02 6201 5168 Fax: 02 6201 5038 Email: [email protected] http://freshwater.canberra.edu.au Printed in September 2000 Growns, Jane. Characterisation of flow in regulated and unregulated streams in eastern Australia. Bibliography. ISBN 1 876810 06 8 Streamflow – Australia, Eastern. I. Cooperative Research Centre for Freshwater Ecology (Australia). II. Title. (Series : Technical report (Cooperative Research Centre for Freshwater Ecology) ; 2000/3). -

Water and Sewerage Services Connection and Supply
Water Services and Sewerage Services Connection and Supply Standard Customer Contract Contents 1 Enquiries and complaints Page 2 2 What obligations do you have? Page 2 Commencement of supply Keep us informed of changes Life support equipment Drinking water and sewerage services Obligations in relation to use of raw water Leasing your premises to others Your equipment Use of fire services Bypassing the meter Other obligations affecting the premises Other customer obligations 3 Your choice of provider for connection work Page 5 4 What standard of service can you expect? Page 6 Continuous supply, quality and pressure not guaranteed Raw water quality Correction of interruptions to your supply Planned interruptions Unplanned interruptions Drought 5 What other rights do you have? Page 7 Ask for identification Are you having difficulties paying your account? Outstanding raw water charges You may request other information When we are permitted to contact you Notices from us Service Failure Rebates Water testing Sewage overflow Plumbing fault in our network Undetected leak Work done by us on your premises ii You may request disconnection 6 Charges Page 10 Schedule of Charges Commencement of charges Unconnected charge Extension and equipment costs Other charges Rebates and concessions Payment of accounts Errors in accounts Disputed accounts GST 7 Can you be required to provide a security Page 13 deposit? 8 Measuring your water consumption Page 13 Meter reading and inspections Check readings Installation -

Gazetteer of West Virginia
Bulletin No. 233 Series F, Geography, 41 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIKECTOU A GAZETTEER OF WEST VIRGINIA I-IEISTRY G-AN3STETT WASHINGTON GOVERNMENT PRINTING OFFICE 1904 A» cl O a 3. LETTER OF TRANSMITTAL. DEPARTMENT OP THE INTEKIOR, UNITED STATES GEOLOGICAL SURVEY, Washington, D. C. , March 9, 190Jh SIR: I have the honor to transmit herewith, for publication as a bulletin, a gazetteer of West Virginia! Very respectfully, HENRY GANNETT, Geogwvpher. Hon. CHARLES D. WALCOTT, Director United States Geological Survey. 3 A GAZETTEER OF WEST VIRGINIA. HENRY GANNETT. DESCRIPTION OF THE STATE. The State of West Virginia was cut off from Virginia during the civil war and was admitted to the Union on June 19, 1863. As orig inally constituted it consisted of 48 counties; subsequently, in 1866, it was enlarged by the addition -of two counties, Berkeley and Jeffer son, which were also detached from Virginia. The boundaries of the State are in the highest degree irregular. Starting at Potomac River at Harpers Ferry,' the line follows the south bank of the Potomac to the Fairfax Stone, which was set to mark the headwaters of the North Branch of Potomac River; from this stone the line runs due north to Mason and Dixon's line, i. e., the southern boundary of Pennsylvania; thence it follows this line west to the southwest corner of that State, in approximate latitude 39° 43i' and longitude 80° 31', and from that corner north along the western boundary of Pennsylvania until the line intersects Ohio River; from this point the boundary runs southwest down the Ohio, on the northwestern bank, to the mouth of Big Sandy River. -

Education Newsletter
TERM 4 2020 Education Newsletter BOOK NOW National Water Week Book a FREE digital education class session with the ACT water cycle model The 19-25 October 2020 is ‘Let’s reimagine our water future,’. The Australian Water Association has put Learn all about the amazing journey of water in the ACT. together a range events, competitions and education Find out how Icon Water delivers water to your tap and material to celebrate the week. Check out their website to treats the sewage from your home and school! get involved at awa.asn.au/nationalwaterweek. Contact us at [email protected] to organise a live webinar education session for your class featuring Calling all creative students! Icon Water have teamed up creative direction from any of with Floriade to launch a Photo the themes below: Comp. The competition is open to photographers of all abilities, 1. Water – our most precious with an under 18s prize resource available. Student participation 2. Water – the environment is encouraged. and/or animals The competition is open to all 3. Floriade blooms in ACT and Queanbeyan Palerang Canberra. residents. There is $5,000 in cash prizes up for grabs. Submissions close Saturday, 31 October. All students need to do is to For more information visit submit a photograph (maximum iconwater.com.au/photocomp of 3 photos per person) using E [email protected] T (02) 6248 3111 Let’s connect iconwater.com.au PRIMARY 2-6 ACTIVITY SECONDARY 7-10 ACTIVITY What is a catchment A handy’ ma Grow your own mini catchment Toilet paper Think of your hand as a map of a catchment.