The Diversification of Amphibians in the Neotropical Savannas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

01-Marty 148.Indd
Copy proofs - 2-12-2013 Bull. Soc. Herp. Fr. (2013) 148 : 419-424 On the occurrence of Dendropsophus leali (Bokermann, 1964) (Anura; Hylidae) in French Guiana by Christian MARTY (1)*, Michael LEBAILLY (2), Philippe GAUCHER (3), Olivier ToSTAIN (4), Maël DEWYNTER (5), Michel BLANC (6) & Antoine FOUQUET (3). (1) Impasse Jean Galot, 97354 Montjoly, Guyane française [email protected] (2) Health Center, 97316, Antécum Pata, Guyane française (3) CNRS Guyane USR 3456, Immeuble Le Relais, 2 avenue Gustave Charlery, 97300 Cayenne, Guyane française (4) Ecobios, BP 44, 97321, Cayenne CEDEX , Guyane française (5) Biotope, Agence Amazonie-Caraïbes, 30 domaine de Montabo, Lotissement Ribal, 97300 Cayenne, Guyane française (6) Pointe Maripa, RN2/PK35, Roura, Guyane française Summary – Dendropsophus leali is a small Amazonian tree frog occurring in Brazil, Peru, Bolivia and Colombia where it mostly inhabits patches of open habitat and disturbed forest. We herein report five new records of this species from French Guiana extending its range 650 km to the north-east and sug- gesting that D. leali could be much more widely distributed in Amazonia than previously thought. The origin of such a disjunct distribution pattern probably lies in historical fluctuations of the forest cover during the late Tertiary and the Quaternary. Poor understanding of Amazonian species distribution still impedes comprehensive investigation of the processes that have shaped Amazonian megabiodiversity. Key-words: Dendropsophus leali, Anura, Hylidae, distribution, French Guiana. Résumé – À propos de la présence de Dendropsophus leali (Bokermann, 1964) (Anura ; Hylidae) en Guyane française. Dendropsophus leali est une rainette de petite taille présente au Brésil, au Pérou, en Bolivie et en Colombie où elle occupe principalement des habitats ouverts ou des forêts perturbées. -

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Natural History of the Tropical Gecko Phyllopezus Pollicaris (Squamata, Phyllodactylidae) from a Sandstone Outcrop in Central Brazil
Herpetology Notes, volume 5: 49-58 (2012) (published online on 18 March 2012) Natural history of the tropical gecko Phyllopezus pollicaris (Squamata, Phyllodactylidae) from a sandstone outcrop in Central Brazil. Renato Recoder1*, Mauro Teixeira Junior1, Agustín Camacho1 and Miguel Trefaut Rodrigues1 Abstract. Natural history aspects of the Neotropical gecko Phyllopezus pollicaris were studied at Estação Ecológica Serra Geral do Tocantins, in the Cerrado region of Central Brazil. Despite initial prospection at different types of habitats, all individuals were collected at sandstone outcrops within savannahs. Most individuals were observed at night, but several specimens were found active during daytime. Body temperatures were significantly higher in day-active individuals. We did not detect sexual dimorphism in size, shape, weight, or body condition. All adult males were reproductively mature, in contrast to just two adult females (11%), one of which contained two oviductal eggs. Dietary data indicates that P. pollicaris feeds upon a variety of arthropods. Dietary overlap between sexes and age classes was moderate to high. The rate of caudal autotomy varied between age classes but not between sexes. Our data, the first for a population ofP. pollicaris from a savannah habitat, are in overall agreement with observations made in populations from Caatinga and Dry Forest, except for microhabitat use and reproductive cycle. Keywords. Cerrado, lizard, local variation, niche breadth, thermal ecology, sexual dimorphism, tail autotomy. Introduction information about aspects of the natural history (habitat Phyllopezus pollicaris (Spix, 1825) is a large-sized, use, morphology, diet, temperatures, reproductive nocturnal and insectivorous gecko native to central condition and caudal autotomy) of a population of South America (Rodrigues, 1986; Vanzolini, Costa P. -

“Relações Evolutivas Entre Ecologia E Morfologia Serpentiforme Em Espécies De Lagartos
UNIVERSIDADE DE SÃO PAULO FFCLRP - DEPARTAMENTO DE BIOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA “Relações evolutivas entre ecologia e morfologia serpentiforme em espécies de lagartos microteiídeos (Sauria: Gymnophthalmidae)”. Mariana Bortoletto Grizante Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da USP, como parte das exigências para a obtenção do título de Mestre em Ciências, Área: Biologia Comparada. Ribeirão Preto 2009 UNIVERSIDADE DE SÃO PAULO FFCLRP - DEPARTAMENTO DE BIOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA “Relações evolutivas entre ecologia e morfologia serpentiforme em espécies de lagartos microteiídeos (Sauria: Gymnophthalmidae)”. Mariana Bortoletto Grizante Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da USP, como parte das exigências para a obtenção do título de Mestre em Ciências, Área: Biologia Comparada. Orientadora: Profª. Drª. Tiana Kohlsdorf Ribeirão Preto 2009 “Relações evolutivas entre ecologia e morfologia serpentiforme em espécies de lagartos microteiídeos (Sauria: Gymnophthalmidae)”. Mariana Bortoletto Grizante Ribeirão Preto, _______________________________ de 2009. _________________________________ _________________________________ Prof(a). Dr(a). Prof(a). Dr(a). _________________________________ _________________________________ Prof(a). Dr(a). Prof(a). Dr(a). _________________________________ Prof ª. Drª. Tiana Kohlsdorf (Orientadora) Dedicado a Antonio Bortoletto e Vainer Grisante, exemplos de curiosidade e perseverança diante dos desafios. AGRADECIMENTOS Realizar esse trabalho só se tornou possível graças à colaboração e ao incentivo de muitas pessoas. A elas, agradeço e com elas, divido a alegria de concluir este trabalho. À Tiana, pela orientação, entusiasmo e confiança, pelas oportunidades, pela amizade, e pelo que ainda virá. Por fazer as histórias terem pé e cabeça! À FAPESP, pelo financiamento desse projeto (processo 2007/52204-8). -

Sexual Dimorphism and Natural History of the Western Mexico Whiptail, Aspidoscelis Costata (Squamata: Teiidae), from Isla Isabel, Nayarit, Mexico
NORTH-WESTERN JOURNAL OF ZOOLOGY 10 (2): 374-381 ©NwjZ, Oradea, Romania, 2014 Article No.: 141506 http://biozoojournals.ro/nwjz/index.html Sexual dimorphism and natural history of the Western Mexico Whiptail, Aspidoscelis costata (Squamata: Teiidae), from Isla Isabel, Nayarit, Mexico Raciel CRUZ-ELIZALDE1, Aurelio RAMÍREZ-BAUTISTA1, *, Uriel HERNÁNDEZ-SALINAS1,2, Cynthia SOSA-VARGAS3, Jerry D. JOHNSON4 and Vicente MATA-SILVA4 1. Centro de Investigaciones Biológicas (CIB), Universidad Autónoma del Estado de Hidalgo, Carretera Pachuca-Tulancingo, Km 4.5 s/n, Colonia Carboneras, Mineral de La Reforma, A.P. 1-69 Plaza Juárez, C.P. 42001, Hidalgo, México. 2. Instituto Politécnico Nacional, CIIDIR Unidad Durango, Sigma 119, Fraccionamiento 20 de Noviembre II, Durango, Durango 34220, México. 3. Laboratorio de Herpetología, Escuela de Biología, Benemérita Universidad Autónoma de Puebla, C. U. Boulevard Valsequillo y Av. San Claudio, Edif. 76, CP. 72570, Puebla, Puebla, México. 4. Department of Biological Sciences, University of Texas at El Paso, 500 West University Avenue, El Paso, TX 79968, USA. *Corresponding author, A. Ramírez-Bautista, E-mail: [email protected] Received: 25. April 2014 / Accepted: 13. June 2014 / Available online: 16. October 2014 / Printed: December 2014 Abstract. Lizard populations found in insular environments may show ecological and morphological characteristics that differ from those living in continents, as a result of different ecological and evolutionary processes. In this study, we analyzed sexual dimorphism, reproduction, and diet in a population of the whiptail lizard, Aspidoscelis costata, from Isla Isabel, Nayarit, Mexico, sampled in 1977 and 1981. Males and females from Isla Isabel showed no sexual dimorphism in many morphological structures, such as snout-vent length (SVL), but they did in femur length (FL) and tibia length (TL). -

Taxonomic Hypotheses and the Biogeography of Speciation in the Tiger Whiptail Complex (Aspidoscelis Tigris: Squamata, Teiidae)
a Frontiers of Biogeography 2021, 13.2, e49120 Frontiers of Biogeography RESEARCH ARTICLE the scientific journal of the International Biogeography Society Taxonomic hypotheses and the biogeography of speciation in the Tiger Whiptail complex (Aspidoscelis tigris: Squamata, Teiidae) Tyler K. Chafin1* , Marlis R. Douglas1 , Whitney J.B. Anthonysamy1,2, Brian K. Sullivan3, James M. Walker1, James E. Cordes4, and Michael E. Douglas1 1 Department of Biological Sciences, University of Arkansas, Fayetteville, Arkansas, 72701, USA; 2 St. Louis College of Pharmacy, 4588 Parkview Place, St. Louis, Missouri, 63110, USA; 3 School of Mathematical and Natural Sciences, P. O. Box 37100, Arizona State University, Phoenix, Arizona, 85069, USA; 4 Division of Mathematics and Sciences, Louisiana State University, Eunice, Louisiana, 70535, USA. *Corresponding author: Tyler K. Chafin, [email protected] Abstract Highlights Biodiversity in southwestern North America has a complex 1. Phylogeographies of vertebrates within the biogeographic history involving tectonism interspersed southwestern deserts of North America have been with climatic fluctuations. This yields a contemporary shaped by climatic fluctuations imbedded within pattern replete with historic idiosyncrasies often difficult broad geomorphic processes. to interpret when viewed from through the lens of modern ecology. The Aspidoscelis tigris (Tiger Whiptail) 2. The resulting synergism drives evolutionary processes, complex (Squamata: Teiidae) is one such group in which such as an expansion of within-species genetic taxonomic boundaries have been confounded by a series divergence over time. Taxonomic inflation often of complex biogeographic processes that have defined results (i.e., an increase in recognized taxa due to the evolution of the clade. To clarify this situation, arbitrary delineations), such as when morphological we first generated multiple taxonomic hypotheses, divergences fail to juxtapose with biogeographic which were subsequently tested using mitochondrial hypotheses. -
For Review Only
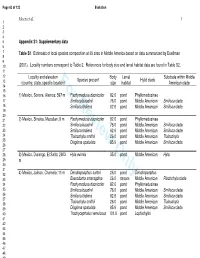
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Dedicated to the Conservation and Biological Research of Costa Rican Amphibians”
“Dedicated to the Conservation and Biological Research of Costa Rican Amphibians” A male Crowned Tree Frog (Anotheca spinosa) peering out from a tree hole. 2 Text by: Brian Kubicki Photography by: Brian Kubicki Version: 3.1 (October 12th, 2009) Mailing Address: Apdo. 81-7200, Siquirres, Provincia de Limón, Costa Rica Telephone: (506)-8889-0655, (506)-8841-5327 Web: www.cramphibian.com Email: [email protected] Cover Photo: Mountain Glass Frog (Sachatamia ilex), Quebrada Monge, C.R.A.R.C. Reserve. 3 Costa Rica is internationally recognized as one of the most biologically diverse countries on the planet in total species numbers for many taxonomic groups of flora and fauna, one of those being amphibians. Costa Rica has 190 species of amphibians known from within its tiny 51,032 square kilometers territory. With 3.72 amphibian species per 1,000 sq. km. of national territory, Costa Rica is one of the richest countries in the world regarding amphibian diversity density. Amphibians are under constant threat by contamination, deforestation, climatic change, and disease. The majority of Costa Rica’s amphibians are surrounded by mystery in regards to their basic biology and roles in the ecology. Through intense research in the natural environment and in captivity many important aspects of their biology and conservation can become better known. The Costa Rican Amphibian Research Center (C.R.A.R.C.) was established in 2002, and is a privately owned and operated conservational and biological research center dedicated to studying, understanding, and conserving one of the most ecologically important animal groups of Neotropical humid forest ecosystems, that of the amphibians. -

Download PDF (Português)
Biota Neotrop., vol. 9, no. 2 Composição, uso de hábitat e estações reprodutivas das espécies de anuros da floresta de restinga da Estação Ecológica Juréia-Itatins, sudeste do Brasil Patrícia Narvaes1, Jaime Bertoluci2,3 & Miguel Trefaut Rodrigues1 1Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo – USP CP 11461, CEP 05422-970, São Paulo, SP, Brasil e-mails: [email protected], [email protected], http://marcus.ib.usp.br/. 2Departamento de Ciências Biológicas, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo – USP, Av. Pádua Dias, 11, CEP 13418-900, Piracicaba, SP, Brasil. e-mail: [email protected], http://www.lcb.esalq.usp.br/ 3Autor para correspondência: Jaime Bertoluci, email: [email protected] NARVAES, P., BERTOLUCI, J. & RODRIGUES, M.T. Species composition, habitat use and breeding seasons of anurans of the restinga forest of the Estação Ecológica Juréia-Itatins, Southeastern Brazil. Biota Neotrop., 9(2): http://www.biotaneotropica.org.br/v9n2/en/abstract?article+bn02009022009. Abstract: Herein we present data on species composition, habitat use, and calling seasons of anurans from the Restinga forest of the Estação Ecológica Juréia-Itatins, Southeastern Brazil. The study site was visited monthly (3 to 4 days) between February and December 1993, a total of 28 days of field work. Three previously selected puddles were searched for anurans between 6:00 and 10:30 PM, when the number of calling males of each species was estimated and the positions of their calling sites were recorded. Anuran fauna is composed by 20 species, the highest richness ever recorded in a Brazilian restinga habitat. -

Amphibians from the Centro Marista São José Das Paineiras, in Mendes, and Surrounding Municipalities, State of Rio De Janeiro, Brazil
Herpetology Notes, volume 7: 489-499 (2014) (published online on 25 August 2014) Amphibians from the Centro Marista São José das Paineiras, in Mendes, and surrounding municipalities, State of Rio de Janeiro, Brazil Manuella Folly¹ *, Juliana Kirchmeyer¹, Marcia dos Reis Gomes¹, Fabio Hepp², Joice Ruggeri¹, Cyro de Luna- Dias¹, Andressa M. Bezerra¹, Lucas C. Amaral¹ and Sergio P. de Carvalho-e-Silva¹ Abstract. The amphibian fauna of Brazil is one of the richest in the world, however, there is a lack of information on its diversity and distribution. More studies are necessary to increase our understanding of amphibian ecology, microhabitat choice and use, and distribution of species along an area, thereby facilitating actions for its management and conservation. Herein, we present a list of the amphibians found in one remnant area of Atlantic Forest, at Centro Marista São José das Paineiras and surroundings. Fifty-one amphibian species belonging to twenty-five genera and eleven families were recorded: Anura - Aromobatidae (one species), Brachycephalidae (six species), Bufonidae (three species), Craugastoridae (one species), Cycloramphidae (three species), Hylidae (twenty-four species), Hylodidae (two species), Leptodactylidae (six species), Microhylidae (two species), Odontophrynidae (two species); and Gymnophiona - Siphonopidae (one species). Visits to herpetological collections were responsible for 16 species of the previous list. The most abundant species recorded in the field were Crossodactylus gaudichaudii, Hypsiboas faber, and Ischnocnema parva, whereas the species Chiasmocleis lacrimae was recorded only once. Keywords: Anura, Atlantic Forest, Biodiversity, Gymnophiona, Inventory, Check List. Introduction characteristics. The largest fragment of Atlantic Forest is located in the Serra do Mar mountain range, extending The Atlantic Forest extends along a great part of from the coast of São Paulo to the coast of Rio de Janeiro the Brazilian coast (Bergallo et al., 2000), formerly (Ribeiro et al., 2009).