Basic ICD-10-CM/PCS Coding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

CPT® New Codes 2019: Biopsy, Skin
Billing and Coding Update Alexander Miller, M.D. AAD Representative to the AMA CPT Advisory Committee New Skin Biopsy CPT® Codes It’s all about the Technique! SPEAKER: Alexander Miller, M.D. AAD Representative to the AMA -CPT Advisory Committee Chair AAD Health Care Finance Committee Arriving on January 1, 2019 New and Restructured Biopsy Codes Tangential biopsy Punch Biopsy Incisional Biopsy How Did We Get Here? CMS CY 2016 Biopsy codes (11100, 11101 identified as potentially mis-valued; high expenditure RUC Survey sent to AAD Members Specialty survey results are the only tool available to support code values Challenging survey results Survey revealed bimodal data distribution; CPT Codes 11100, 11101 referred to CPT for respondents were valuing different procedures restructuring Rationale for New Codes 11100; 11101 • Previous skin biopsy codes did not distinguish between the different biopsy techniques that were being used CPT Recommended technique specification in new biopsy codes • Will also provide for reimbursement commensurate with the technique used How Did We Get Here? • CPT Editorial Panel deleted 11100; 11101 February 2017 • 6 New codes created based on technique utilized • Each technique: primary code and add-on code March 2017 • RUC survey sent to AAD members April 2017 • Survey results presented to the RUC Biopsy Codes Effective Jan., 1, 2019 • Integumentary biopsy codes 11755 Biopsy of nail unit (plate, bed, matrix, hyponychium, proximal and lateral nail folds 11100, 11101 have been deleted 30100 Biopsy, intranasal • New -

Information for Patients Having a Sigmoid Colectomy
Patient information – Pre-operative Assessment Clinic Information for patients having a sigmoid colectomy This leaflet will explain what will happen when you come to the hospital for your operation. It is important that you understand what to expect and feel able to take an active role in your treatment. Your surgeon will have already discussed your treatment with you and will give advice about what to do when you get home. What is a sigmoid colectomy? This operation involves removing the sigmoid colon, which lies on the left side of your abdominal cavity (tummy). We would then normally join the remaining left colon to the top of the rectum (the ‘storage’ organ of the bowel). The lines on the attached diagram show the piece of bowel being removed. This operation is done with you asleep (general anaesthetic). The operation not only removes the bowel containing the tumour but also removes the draining lymph glands from this part of the bowel. This is sent to the pathologists who will then analyse each bit of the bowel and the lymph glands in detail under the microscope. This operation can often be completed in a ‘keyhole’ manner, which means less trauma to the abdominal muscles, as the biggest wound is the one to remove the bowel from the abdomen. Sometimes, this is not possible, in which case the same operation is done through a bigger incision in the abdominal wall – this is called an ‘open’ operation. It does take longer to recover with an open operation but, if it is necessary, it is the safest thing to do. -

Reduction Mammoplasty
Reduction Mammoplasty Date of Origin: 02/1999 Last Review Date: 09/23/2020 Effective Date: 10/01/2020 Dates Reviewed: 05/1999, 10/2000, 09/2001, 03/2002, 05/2002, 08/2002, 10/2003, 10/2004, 09/2005, 11/2005, 01/2006, 02/2007, 02/2008, 02/2009, 07/2010, 02/2011, 01/2012, 10/2012, 08/2013, 07/2014, 10/2014, 12/2015, 05/2016, 06/2017, 08/2018, 02/2019, 09/2019, 09/2020 Developed By: Medical Necessity Criteria Committee I. Description A breast reduction, or reduction mammoplasty, is a surgical excision of a substantial portion of the breast including the skin and underlying glandular tissue, that reduces the size, changes the shape and/or lifts the breast tissue. Reduction mammoplasty may be approved on an individual basis when medical necessity has been established to relieve a physical functional impairment of members who are 16 years of age or older who have reached physical maturity. Reduction mammoplasty for cosmetic reasons is not a covered benefit. A reduction mammoplasty that is part of a reconstructive procedure related to breast cancer is not considered in this policy; See Moda Health Breast Reconstruction criteria. II. Criteria: CWQI HCS-0058A A. Reduction mammoplasty will be covered to plan limitations when ALL of the following criteria are met: a. The patient must be at least age 16 or older and/or Tanner stage V of Tanner staging of sexual maturity (See Addendum I for Tanner Staging) and ALL of the following: i. Patient’s weight has not changed in the past two years or has stabilized. -
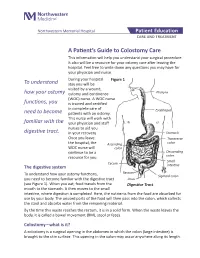
A Patient's Guide to Colostomy Care
Northwestern Memorial Hospital Patient Education CARE AND TREATMENT A Patient’s Guide to Colostomy Care This information will help you understand your surgical procedure. It also will be a resource for your ostomy care after leaving the hospital. Feel free to write down any questions you may have for your physician and nurse. During your hospital Figure 1 To understand stay you will be visited by a wound, how your ostomy ostomy and continence Pharynx (WOC) nurse. A WOC nurse functions, you is trained and certified in complete care of Esophagus need to become patients with an ostomy. This nurse will work with familiar with the your physician and staff nurses to aid you digestive tract. in your recovery. Stomach Once you leave Transverse the hospital, the Ascending colon WOC nurse will colon continue to be a Descending resource for you. colon Small Cecum The digestive system intestine Rectum To understand how your ostomy functions, Sigmoid colon you need to become familiar with the digestive tract Anus (see Figure 1). When you eat, food travels from the Digestive Tract mouth to the stomach. It then moves to the small intestine, where digestion is completed. Here, the nutrients from the food are absorbed for use by your body. The unused parts of the food will then pass into the colon, which collects the stool and absorbs water from the remaining material. By the time this waste reaches the rectum, it is in a solid form. When the waste leaves the body, it is called a bowel movement (BM), stool or feces. -

An Evaluation of 100 Symptomatic Women with Breast Implants Or Silicone Fluid Injections
ORIGINAL ARTICLE Adjuvant Breast Disease: An Evaluation of 100 Symptomatic Women with Breast Implants or Silicone Fluid Injections Britta Ostermeyer Shoaib, Bernard M Patten and Dick S Calkins1 Department of Neurology, Baylor College of Medicine and 1Krug Life Science, Houston, TX, USA (Receivedfor publicationon December7, 1993) Abstract. We evaluated 100 referred women with breast implants (n=97) or silicone fluid injections (n=3) into breasts who developed various symptoms. All reported symptoms occurred at a median latency period of 6 years (range 0-24 years) after implantation or injection of silicone. Commonest symptoms were weakness (95%), fatigability (95%), myalgia (90%), morning stiffness (89%), arthralgia (81%), memory loss (81%), sensory loss (77%), headache (73%) and dry eyes and dry mouth (72%). Laboratory results revealed abnormal levels of serum immunoglobulins or complement in 57% and autoantibodies in 78%. Sural nerve biopsy was abnormal in 80% with the major finding of loss of myelinated fibers in 79%. Biceps muscle biopsy was abnormal in 58% with the major finding of neurogenic atrophy in 27%. Ninety six patients underwent implant removal; 60% of the patients were found to have one or both implants ruptured with silicone spilled into tissue. At time of removal, a pectoralis major muscle biopsy was taken which was abnormal in 89% with the major finding of neurogenic atrophy in 55%. Biopsy of implant capsule was abnormal in 94% showing foreign body giant cells containing retractile material consistent with silicone in 69% whether or not the elastomer shell was ruptured. Silicone can cause a systemic autoimmune disease with a variety of symptoms probably due to a global activation of the immune system. -

ANMC Specialty Clinic Services
Cardiology Dermatology Diabetes Endocrinology Ear, Nose and Throat (ENT) Gastroenterology General Medicine General Surgery HIV/Early Intervention Services Infectious Disease Liver Clinic Neurology Neurosurgery/Comprehensive Pain Management Oncology Ophthalmology Orthopedics Orthopedics – Back and Spine Podiatry Pulmonology Rheumatology Urology Cardiology • Cardiology • Adult transthoracic echocardiography • Ambulatory electrocardiology monitor interpretation • Cardioversion, electrical, elective • Central line placement and venous angiography • ECG interpretation, including signal average ECG • Infusion and management of Gp IIb/IIIa agents and thrombolytic agents and antithrombotic agents • Insertion and management of central venous catheters, pulmonary artery catheters, and arterial lines • Insertion and management of automatic implantable cardiac defibrillators • Insertion of permanent pacemaker, including single/dual chamber and biventricular • Interpretation of results of noninvasive testing relevant to arrhythmia diagnoses and treatment • Hemodynamic monitoring with balloon flotation devices • Non-invasive hemodynamic monitoring • Perform history and physical exam • Pericardiocentesis • Placement of temporary transvenous pacemaker • Pacemaker programming/reprogramming and interrogation • Stress echocardiography (exercise and pharmacologic stress) • Tilt table testing • Transcutaneous external pacemaker placement • Transthoracic 2D echocardiography, Doppler, and color flow Dermatology • Chemical face peels • Cryosurgery • Diagnosis -

Therapeutic Mammaplasty Information for Patients the Aim of This Booklet Is to Give You Some General Information About Your Surgery
Oxford University Hospitals NHS Trust Therapeutic mammaplasty Information for patients The aim of this booklet is to give you some general information about your surgery. If you have any questions or concerns after reading it please discuss them with your breast care nurse practitioner or a member of staff at the Jane Ashley Centre. Telephone numbers are given at the end of this booklet. Author: Miss P.G.Roy, Consultant Oncoplastic Breast Surgeon Oxford University Hospitals NHS Trust Oxford OX3 9DU page 2 Therapeutic mammaplasty This operation involves combining a wide local excision (also known as a lumpectomy) with a breast reduction technique resulting in a smaller, uplifted and better shaped breast. This means that the lump can be removed with a wide rim of healthy tissue. The nipple and areola are preserved with their intact blood supply and the remaining breast tissue is repositioned to allow reshaping of the breast. The scars are either in the shape of a lollipop or an anchor (as shown below). You may have a drain placed in the wound to remove excess fluid; this is usually left in for 24 hours. This procedure can be carried out on one or both of your breasts, as discussed with your surgeon. Vertical mammaplasty Lollipop scar Wise pattern Anchor shaped scar mammaplasty page 3 Your nipple is moved to a new position to suit your new breast shape and size but it may end up in a position different to your wishes. The surgeon will try to achieve a mutually agreed breast size whilst performing the operation; however a cup size cannot be guaranteed and there are likely to be further significant changes to your breast after radiotherapy. -

42 CFR Ch. IV (10–1–12 Edition) § 410.35
§ 410.35 42 CFR Ch. IV (10–1–12 Edition) the last screening mammography was (1) Colorectal cancer screening tests performed. means any of the following procedures furnished to an individual for the pur- [59 FR 49833, Sept. 30, 1994, as amended at 60 FR 14224, Mar. 16, 1995; 60 FR 63176, Dec. 8, pose of early detection of colorectal 1995; 62 FR 59100, Oct. 31, 1997; 63 FR 4596, cancer: Jan. 30, 1998] (i) Screening fecal-occult blood tests. (ii) Screening flexible § 410.35 X-ray therapy and other radi- sigmoidoscopies. ation therapy services: Scope. (iii) In the case of an individual at Medicare Part B pays for X-ray ther- high risk for colorectal cancer, screen- apy and other radiation therapy serv- ing colonoscopies. ices, including radium therapy and ra- (iv) Screening barium enemas. dioactive isotope therapy, and mate- (v) Other tests or procedures estab- rials and the services of technicians ad- lished by a national coverage deter- ministering the treatment. mination, and modifications to tests [51 FR 41339, Nov. 14, 1986. Redesignated at 55 under this paragraph, with such fre- FR 53522, Dec. 31, 1990] quency and payment limits as CMS de- termines appropriate, in consultation § 410.36 Medical supplies, appliances, with appropriate organizations and devices: Scope. (2) Screening fecal-occult blood test (a) Medicare Part B pays for the fol- means— lowing medical supplies, appliances (i) A guaiac-based test for peroxidase and devices: activity, testing two samples from (1) Surgical dressings, and splints, each of three consecutive stools, or, casts, and other devices used for reduc- (ii) Other tests as determined by the tion of fractures and dislocations. -

A Clinical and Histological Study of Radiofrequency-Assisted Liposuction (RFAL) Mediated Skin Tightening and Cellulite Improvement ——RFAL for Skin Tightening
Journal of Cosmetics, Dermatological Sciences and Applications, 2011, 1, 36-42 doi:10.4236/jcdsa.2011.12006 Published Online June 2011 (http://www.SciRP.org/journal/jcdsa) A Clinical and Histological Study of Radiofrequency-Assisted Liposuction (RFAL) Mediated Skin Tightening and Cellulite Improvement ——RFAL for Skin Tightening Marc Divaris1, Sylvie Boisnic2, Marie-Christine Branchet2, Malcolm D. Paul3 1Plastic and Maxillo-Facial Surgery, University of Pitie Salpetiere, Paris, France; 2Institution GREDECO, Paris, France; 3Department of Surgery, Aesthetic and PlasticSurgery Institute, University of California, Irvine, USA. Email: [email protected] Received May 1st, 2011; revised May 27th, 2011; accepted June 6th, 2011. ABSTRACT Background: A novel Radiofrequency-Assisted Liposuction (RFAL) technology was evaluated clinically. Parallel origi- nal histological studies were conducted to substantiate the technology’s efficacy in skin tightening, and cellulite im- provement. Methods: BodyTiteTM system, utilizing the RFAL technology, was used for treating patients on abdomen, hips, flanks and arms. Clinical results were measured on 53 patients up to 6 months follow-up. Histological and bio- chemical studies were conducted on 10 donors by using a unique GREDECO model of skin fragments cultured under survival conditions. Fragments from RFAL treated and control areas were examined immediately and after 10 days in culture, representing long-term results. Skin fragments from patients with cellulite were also examined. Results: Grad- ual improvement in circumference reduction (3.9 - 4.9 cm) and linear contraction (8% - 38%) was observed until the third month. These results stabilized at 6 months. No adverse events were recorded. Results were graded as excellent by most patients, including the satisfaction from minimal pain, bleeding, and downtime. -

Co™™I™™Ee Opinion
The American College of Obstetricians and Gynecologists WOMEN’S HEALTH CARE PHYSICIANS COMMITTEE OPINION Number 673 • September 2016 (Replaces Committee Opinion No. 345, October 2006) Committee on Gynecologic Practice This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and the American Society for Colposcopy and Cervical Pathology (ASCCP) in collaboration with committee member Ngozi Wexler, MD, MPH, and ASCCP members and experts Hope K. Haefner, MD, Herschel W. Lawson, MD, and Colleen K. Stockdale, MD, MS. This document reflects emerging clinical and scientific advances as of the date issued and is subject to change. The information should not be construed as dictating an exclusive course of treatment or procedure to be followed. Persistent Vulvar Pain ABSTRACT: Persistent vulvar pain is a complex disorder that frequently is frustrating to the patient and the clinician. It can be difficult to treat and rapid resolution is unusual, even with appropriate therapy. Vulvar pain can be caused by a specific disorder or it can be idiopathic. Idiopathic vulvar pain is classified as vulvodynia. Although optimal treatment remains unclear, consider an individualized, multidisciplinary approach to address all physical and emotional aspects possibly attributable to vulvodynia. Specialists who may need to be involved include sexual counselors, clinical psychologists, physical therapists, and pain specialists. Patients may perceive this approach to mean the practitioner does not believe their pain is “real”; thus, it is important to begin any treatment approach with a detailed discussion, including an explanation of the diagnosis and determination of realistic treatment goals. Future research should aim at evaluating a multimodal approach in the treatment of vulvodynia, along with more research on the etiologies of vulvodynia. -
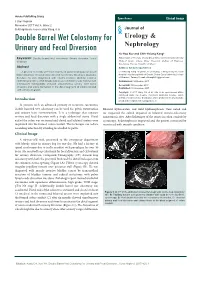
Double Barrel Wet Colostomy for Urinary and Fecal Diversion
Open Access Clinical Image J Urol Nephrol November 2017 Vol.:4, Issue:2 © All rights are reserved by Kang,et al. Journal of Double Barrel Wet Colostomy for Urology & Urinary and Fecal Diversion Nephrology Yu-Hao Xue and Chih-Hsiung Kang* Keywords: Double barrel wet colostomy; Urinary diversion; Fecal Department of Urology, Chang Gung Memorial Hospital-Kaohsiung diversion Medical Center, Chang Gung University College of Medicine, Kaohsiung, Taiwan, Republic of China Abstract Address for Correspondence A 60-year-old male who had a history of spinal cord injury received Chih-Hsiung Kang, Department of Urology, Chang Gung Memorial loop colostomy for fecal diversion and cystostomy for urinary diversion. Hospital - Kaohsiung Medical Center, Chang Gung University College Because he was diagnosed with muscle invasive bladder cancer, of Medicine, Taiwan, E- mail: [email protected] radical cystectomy and double barrel wet colostomy was conducted. Submission: 30 October, 2017 Computed tomography showed simultaneous urinary and fecal Accepted: 06 November, 2017 diversion and stone formation in the distal segment of colon conduit Published: 10 November, 2017 with urinary diversion. Copyright: © 2017 Kang CH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, Introduction provided the original work is properly cited. In patients with an advanced primary or recurrent carcinoma, double-barreled wet colostomy can be used for pelvic exenteration Bilateral hydroureters and mild hydronephrosis were noted and and urinary tract reconstruction. It is a technique that separate we suspected the calculi impacted in bilateral ureteto-colostomy urinary and fecal diversion with a single abdominal stoma.