Gentianaceae in Context
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flowering Plants Eudicots Apiales, Gentianales (Except Rubiaceae)
Edited by K. Kubitzki Volume XV Flowering Plants Eudicots Apiales, Gentianales (except Rubiaceae) Joachim W. Kadereit · Volker Bittrich (Eds.) THE FAMILIES AND GENERA OF VASCULAR PLANTS Edited by K. Kubitzki For further volumes see list at the end of the book and: http://www.springer.com/series/1306 The Families and Genera of Vascular Plants Edited by K. Kubitzki Flowering Plants Á Eudicots XV Apiales, Gentianales (except Rubiaceae) Volume Editors: Joachim W. Kadereit • Volker Bittrich With 85 Figures Editors Joachim W. Kadereit Volker Bittrich Johannes Gutenberg Campinas Universita¨t Mainz Brazil Mainz Germany Series Editor Prof. Dr. Klaus Kubitzki Universita¨t Hamburg Biozentrum Klein-Flottbek und Botanischer Garten 22609 Hamburg Germany The Families and Genera of Vascular Plants ISBN 978-3-319-93604-8 ISBN 978-3-319-93605-5 (eBook) https://doi.org/10.1007/978-3-319-93605-5 Library of Congress Control Number: 2018961008 # Springer International Publishing AG, part of Springer Nature 2018 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -

Recovery Plan for Tyoj5llllt . I-Bland Plants
Recovery Plan for tYOJ5llllt. i-bland Plants RECOVERY PLAN FOR MULTI-ISLAND PLANTS Published by U.S. Fish and Wildlife Service Portland, Oregon Approved: Date: / / As the Nation’s principal conservation agency, the Department of the Interior has responsibility for most ofour nationally owned public lands and natural resources. This includes fostering the wisest use ofour land and water resources, protecting our fish and wildlife, preserving the environmental and cultural values ofour national parks and historical places, and providing for the enjoyment of life through outdoor recreation. The Department assesses our energy and mineral resources and works to assure that their development is in the best interests ofall our people. The Department also has a major responsibility for American Indian reservation communities and for people who live in island Territories under U.S. administration. DISCLAIMER PAGE Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance ofrecovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Costs indicated for task implementation and/or time for achievement ofrecovery are only estimates and are subject to change. Recovery plans do not necessarily represent the views nor the official positions or approval ofany individuals or agencies involved in the plan formulation, otherthan the U.S. Fish and Wildlife Service. They represent the official position ofthe U.S. -

Temporal and Spatial Origin of Gesneriaceae in the New World Inferred from Plastid DNA Sequences
bs_bs_banner Botanical Journal of the Linnean Society, 2013, 171, 61–79. With 3 figures Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences MATHIEU PERRET1*, ALAIN CHAUTEMS1, ANDRÉA ONOFRE DE ARAUJO2 and NICOLAS SALAMIN3,4 1Conservatoire et Jardin botaniques de la Ville de Genève, Ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland 2Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, Rua Santa Adélia, 166, Bairro Bangu, Santo André, Brazil 3Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland 4Swiss Institute of Bioinformatics, Quartier Sorge, CH-1015 Lausanne, Switzerland Received 15 December 2011; revised 3 July 2012; accepted for publication 18 August 2012 Gesneriaceae are represented in the New World (NW) by a major clade (c. 1000 species) currently recognized as subfamily Gesnerioideae. Radiation of this group occurred in all biomes of tropical America and was accompanied by extensive phenotypic and ecological diversification. Here we performed phylogenetic analyses using DNA sequences from three plastid loci to reconstruct the evolutionary history of Gesnerioideae and to investigate its relationship with other lineages of Gesneriaceae and Lamiales. Our molecular data confirm the inclusion of the South Pacific Coronanthereae and the Old World (OW) monotypic genus Titanotrichum in Gesnerioideae and the sister-group relationship of this subfamily to the rest of the OW Gesneriaceae. Calceolariaceae and the NW genera Peltanthera and Sanango appeared successively sister to Gesneriaceae, whereas Cubitanthus, which has been previously assigned to Gesneriaceae, is shown to be related to Linderniaceae. Based on molecular dating and biogeographical reconstruction analyses, we suggest that ancestors of Gesneriaceae originated in South America during the Late Cretaceous. -

Native Or Suitable Plants City of Mccall
Native or Suitable Plants City of McCall The following list of plants is presented to assist the developer, business owner, or homeowner in selecting plants for landscaping. The list is by no means complete, but is a recommended selection of plants which are either native or have been successfully introduced to our area. Successful landscaping, however, requires much more than just the selection of plants. Unless you have some experience, it is suggested than you employ the services of a trained or otherwise experienced landscaper, arborist, or forester. For best results it is recommended that careful consideration be made in purchasing the plants from the local nurseries (i.e. Cascade, McCall, and New Meadows). Plants brought in from the Treasure Valley may not survive our local weather conditions, microsites, and higher elevations. Timing can also be a serious consideration as the plants may have already broken dormancy and can be damaged by our late frosts. Appendix B SELECTED IDAHO NATIVE PLANTS SUITABLE FOR VALLEY COUNTY GROWING CONDITIONS Trees & Shrubs Acer circinatum (Vine Maple). Shrub or small tree 15-20' tall, Pacific Northwest native. Bright scarlet-orange fall foliage. Excellent ornamental. Alnus incana (Mountain Alder). A large shrub, useful for mid to high elevation riparian plantings. Good plant for stream bank shelter and stabilization. Nitrogen fixing root system. Alnus sinuata (Sitka Alder). A shrub, 6-1 5' tall. Grows well on moist slopes or stream banks. Excellent shrub for erosion control and riparian restoration. Nitrogen fixing root system. Amelanchier alnifolia (Serviceberry). One of the earlier shrubs to blossom out in the spring. -
Dwarf Thistle, Cirsi
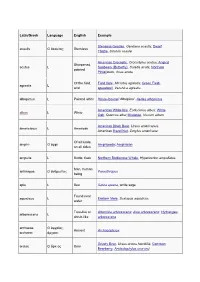
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Sipaneopsis (Sipaneeae, Ixoroideae): a Unique Flowering Process in the Family Rubiaceae and Its Taxonomic Significance
Phytotaxa 302 (1): 040–048 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.302.1.3 Sipaneopsis (Sipaneeae, Ixoroideae): A unique flowering process in the family Rubiaceae and its taxonomic significance PIERO G. DELPRETE1* 1Herbier de Guyane, Institut de Recherche pour le Développement (IRD), UMR AMAP, Boite Postale 90165, 97323 Cayenne Cedex, French Guiana, France. *Author for correspondence: E-mail: [email protected] Abstract The process of anthesis of Sipaneopsis is unique within the Rubiaceae, and is here described and illustrated in detail for the first time. During the initial stage of anthesis the flowers are small, with the corolla lobes already open and erect before tube elongation, and the corolla lobe basal appendages are appressed against each other, forming a convex structure at the corolla mouth obstructing the entrance of visitors and pollinators. At the final stage of anthesis, the corolla lobes reflex and become perpendicular to the tube, and their basal appendages become erect, not touching each other, allowing visitors and pollinators to access the corolla tube and the nectar disk. Neobertiera and Sipaneopsis are unique within the tribe Sipaneeae in having indehiscent dry fruits and recent molecular phylogenies positioned them as sister taxa. Sipaneopsis is distinguished from Neobertiera in having flowers consistently homostylous (vs. distylous or rarely tristylous in Neobertiera), stamens inserted at the middle of the corolla tube (vs. at variable positions), and five triangular appendages at the base of each corolla lobe (vs. corolla lobes thickened at base, without appendages). -

ISB: Atlas of Florida Vascular Plants
Longleaf Pine Preserve Plant List Acanthaceae Asteraceae Wild Petunia Ruellia caroliniensis White Aster Aster sp. Saltbush Baccharis halimifolia Adoxaceae Begger-ticks Bidens mitis Walter's Viburnum Viburnum obovatum Deer Tongue Carphephorus paniculatus Pineland Daisy Chaptalia tomentosa Alismataceae Goldenaster Chrysopsis gossypina Duck Potato Sagittaria latifolia Cow Thistle Cirsium horridulum Tickseed Coreopsis leavenworthii Altingiaceae Elephant's foot Elephantopus elatus Sweetgum Liquidambar styraciflua Oakleaf Fleabane Erigeron foliosus var. foliosus Fleabane Erigeron sp. Amaryllidaceae Prairie Fleabane Erigeron strigosus Simpson's rain lily Zephyranthes simpsonii Fleabane Erigeron vernus Dog Fennel Eupatorium capillifolium Anacardiaceae Dog Fennel Eupatorium compositifolium Winged Sumac Rhus copallinum Dog Fennel Eupatorium spp. Poison Ivy Toxicodendron radicans Slender Flattop Goldenrod Euthamia caroliniana Flat-topped goldenrod Euthamia minor Annonaceae Cudweed Gamochaeta antillana Flag Pawpaw Asimina obovata Sneezeweed Helenium pinnatifidum Dwarf Pawpaw Asimina pygmea Blazing Star Liatris sp. Pawpaw Asimina reticulata Roserush Lygodesmia aphylla Rugel's pawpaw Deeringothamnus rugelii Hempweed Mikania cordifolia White Topped Aster Oclemena reticulata Apiaceae Goldenaster Pityopsis graminifolia Button Rattlesnake Master Eryngium yuccifolium Rosy Camphorweed Pluchea rosea Dollarweed Hydrocotyle sp. Pluchea Pluchea spp. Mock Bishopweed Ptilimnium capillaceum Rabbit Tobacco Pseudognaphalium obtusifolium Blackroot Pterocaulon virgatum -

In Vitro Regeneration and Transformation of Blackstonia Perfoliata
BIOLOGIA PLANTARUM 48 (3): 333-338, 2004 In vitro regeneration and transformation of Blackstonia perfoliata A. BIJELOVIĆ*1, N. ROSIĆ**, J. MILJUŠ-DJUKIĆ***, S. NINKOVIĆ** and D. GRUBIŠIĆ** Institute of Botany, Faculty of Biology, Takovska 43, 11000 Belgrade, Serbia and Montenegro* Institute for Biological Research “Siniša Stanković”, 29. Novembra 142, 11060 Belgrade, Serbia and Montenegro** Institute for Molecular Genetics and Genetic Engineering, Vojvode Stepe 444a, 11000 Belgrade, Serbia and Montenegro*** Abstract In vitro root culture of yellow wort (Blackstonia perfoliata (L.) Huds.) was initiated on Murashige and Skoog (MS) medium. In the presence of benzylaminopurine (BAP) numerous adventitious buds formed, which developed into shoots. Presence of indole-3-butyric acid (IBA) in media significantly decreased number of buds, but increased development of lateral roots. On hormone-free medium shoots successfully rooted and developed flowers and viable seeds that formed another generation. Shoot cultures of B. perfoliata inoculated with suspension of Agrobacterium rhizogenes strain A4M70GUS developed hairy roots at 3 weeks and they were cultured on hormone-free MS medium. Spontaneous shoot regeneration occurred in 3 clones. Additional key words: Agrobacterium rhizogenes, hairy roots, regeneration, root culture. Introduction Blackstonia perfoliata (yellow wort) (L.) Huds. (Chlora 1997a, Menković et al. 1998, Vintehalter and Vinterhalter perfoliata L., Gentiana perfoliata L., Seguiera perfoliata 1998, Mikula and Rybczynski 2001). O. Kuntze), Gentianaceae, is an annual plant, 10 - 60 cm Since B. perfoliata could be used in medicine instead high, with long internodes, triangular leaves, sometimes of Radix Gentianae, this plant can be produced in great narrowing towards the base (Jovanović-Dunjić 1973). It biomass in culture in vitro. -

9 Costion Plant Endemism 133-166 PROOFS
Micronesica 41(1): 131–164, 2009 Plant Endemism, Rarity, and Threat in Palau, Micronesia: A Geographical Checklist and Preliminary Red List Assessment 1 CRAIG M. COSTION Department of Ecology and Evolutionary Biology, School of Earth and Environmental Sciences, University of Adelaide, Adelaide SA 5001 [email protected] ANN HILLMANN KITALONG The Environment, Inc., P.O. Box 1696, Koror, Palau 96940 TARITA HOLM Palau Conservation Society/PALARIS, P.O. Box 1811, Koror, Palau, 96940 Abstract—An official checklist of the endemic plant species of Palau has been long awaited, and is presented here for the first time. For each species a substrate limitation, growth form, and relative abundance is listed. In addition an IUCN red list assessment was conducted using all available data. For over half of the endemic species there is insufficient data to provide a red listing status however an expected minimum number of threatened plants out of the total is inferred. Approximately 15% of Palau’s endemic plants are believed to be only known from the type collection and many more only known from a few collections. These taxa however may now be prioritized and targeted for future inventory and research. The taxonomic robustness of several of these taxa is questionable and it is expected that more endemic species will be lost to synonymy in the future. Previous estimations have significantly over-estimated the rate of plant endemism in Palau (e.g., 194). Here, 130 plants are recognized for Palau, making its level of plant endem- ism comparable to some of its neighboring Micronesian islands to the east, notably Guam and Pohnpei. -

The Island Rule and Its Application to Multiple Plant Traits
The island rule and its application to multiple plant traits Annemieke Lona Hedi Hendriks A thesis submitted to the Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity Victoria University of Wellington, New Zealand 2019 ii “The larger the island of knowledge, the longer the shoreline of wonder” Ralph W. Sockman. iii iv General Abstract Aim The Island Rule refers to a continuum of body size changes where large mainland species evolve to become smaller and small species evolve to become larger on islands. Previous work focuses almost solely on animals, with virtually no previous tests of its predictions on plants. I tested for (1) reduced floral size diversity on islands, a logical corollary of the island rule and (2) evidence of the Island Rule in plant stature, leaf size and petiole length. Location Small islands surrounding New Zealand; Antipodes, Auckland, Bounty, Campbell, Chatham, Kermadec, Lord Howe, Macquarie, Norfolk, Snares, Stewart and the Three Kings. Methods I compared the morphology of 65 island endemics and their closest ‘mainland’ relative. Species pairs were identified. Differences between archipelagos located at various latitudes were also assessed. Results Floral sizes were reduced on islands relative to the ‘mainland’, consistent with predictions of the Island Rule. Plant stature, leaf size and petiole length conformed to the Island Rule, with smaller plants increasing in size, and larger plants decreasing in size. Main conclusions Results indicate that the conceptual umbrella of the Island Rule can be expanded to plants, accelerating understanding of how plant traits evolve on isolated islands.