Portuguese Lavenders Evaluation of Their Potential Use For
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Catalogue of Coleoptera Specimens with Potential Forensic Interest in the Goulandris Natural History Museum Collection
ENTOMOLOGIA HELLENICA Vol. 25, 2016 A catalogue of Coleoptera specimens with potential forensic interest in the Goulandris Natural History Museum collection Dimaki Maria Goulandris Natural History Museum, 100 Othonos St. 14562 Kifissia, Greece Anagnou-Veroniki Maria Makariou 13, 15343 Aghia Paraskevi (Athens), Greece Tylianakis Jason Zoology Department, University of Canterbury, Private Bag 4800, Christchurch, New Zealand http://dx.doi.org/10.12681/eh.11549 Copyright © 2017 Maria Dimaki, Maria Anagnou- Veroniki, Jason Tylianakis To cite this article: Dimaki, M., Anagnou-Veroniki, M., & Tylianakis, J. (2016). A catalogue of Coleoptera specimens with potential forensic interest in the Goulandris Natural History Museum collection. ENTOMOLOGIA HELLENICA, 25(2), 31-38. doi:http://dx.doi.org/10.12681/eh.11549 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 27/12/2018 06:22:38 | ENTOMOLOGIA HELLENICA 25 (2016): 31-38 Received 15 March 2016 Accepted 12 December 2016 Available online 3 February 2017 A catalogue of Coleoptera specimens with potential forensic interest in the Goulandris Natural History Museum collection MARIA DIMAKI1’*, MARIA ANAGNOU-VERONIKI2 AND JASON TYLIANAKIS3 1Goulandris Natural History Museum, 100 Othonos St. 14562 Kifissia, Greece 2Makariou 13, 15343 Aghia Paraskevi (Athens), Greece 3Zoology Department, University of Canterbury, Private Bag 4800, Christchurch, New Zealand ABSTRACT This paper presents a catalogue of the Coleoptera specimens in the Goulandris Natural History Museum collection that have potential forensic interest. Forensic entomology can help to estimate the time elapsed since death by studying the necrophagous insects collected on a cadaver and its surroundings. In this paper forty eight species (369 specimens) are listed that belong to seven families: Silphidae (3 species), Staphylinidae (6 species), Histeridae (11 species), Anobiidae (4 species), Cleridae (6 species), Dermestidae (14 species), and Nitidulidae (4 species). -

Evaluation of Antimicrobial Activity and Activity on the Autonomic Nervous
Progress in Nutrition 2019; Vol. 21, N. 3: 584-590 DOI: 10.23751/pn.v21i3.8385 © Mattioli 1885 Original article Evaluation of antimicrobial activity and activity on the autonomic nervous system of the lavender essential oils from Montenegro Svetlana Perovic1, Snezana Pantovic2, Valentina Scepanovic1, Andrej Perovic1, Vladimir Zivkovic3, Biljana Damjanovic-Vratnica4 1Department of Biology, Faculty of Natural Science and Mathematics, University of Montenegro, Dz. Vasingtona bb, Podgorica 2Faculty of Medicine, University of Montenegro, Krusevac bb, Podgorica - E-mail: [email protected]; 3Center for Eco- toxicological Research Podgorica, Boulevard Sarla de Gola 2, Podgorica; 4Faculty of Metallurgy and Technology, University of Montenegro, Dz. Vasingtona bb, Podgorica Summary. This study investigates chemical composition and antimicrobial activity of essential oil of Lavandula officinalis from Montenegro, Mediterranean, and effects on autonomic nervous system. The oil was analysed by GC-MS technique in order to determine the majority compounds while dilution method was used to determine minimal inhibitory concentration. The present study assessed autonomic parameters such as blood pressure, heart rate, and skin temperature to determine the arousal level of the autonomic nervous system. In addition, subjects were asked to estimate their mood responses such as feeling pleasant or unpleasant, uncomfortable, sen- suality, relaxation, or refreshing in order to assess subjective behavioural arousal. Chemical analysis by GC-MS identified 31 compounds in lavender oil representing 96.88% of the total oil. Linalool (24.84%), was a major component, together with linalyl acetate (22.39%), 1,8 cineole (18.13%) and camphor (12.88%). The investigat- ed lavender oil consisted mostly of oxygenated monoterpenes (87.95%) and monoterpene hydrocarbons (7%). -

Forest Fringe Communities of the Southwestern Iberian Peninsula
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade do Algarve Scientific article http://dx.doi.org/10.5154/r.rchscfa.2017.12.072 Forest fringe communities of the southwestern Iberian Peninsula Comunidades de orla forestal en el suroeste de la península ibérica Ricardo Quinto-Canas1,2*; Paula Mendes3; Ana Cano-Ortiz4; Carmelo Maria Musarella4,5; Carlos Pinto-Gomes3 1Universidade do Algarve, Faculdade de Ciências e Tecnologia. Campus de Gambelas, 8005-139. Faro, Portugal. 2CCMAR – Centro de Ciências do Mar, Universidade do Algarve, Campus de Gambelas, 8005-139 Faro, Portugal. 3Universidade de Évora, Escola de Ciência e Tecnologia, Instituto de Ciências Agrárias e Ambientais Mediterrânicas (ICAAM), Departamento de Paisagem, Ambiente e Ordenamento. Rua 25 Romão Ramalho, nº 59, P-7000-671. Évora, Portugal. 4Universidad de Jaén, Depto. de Biología Animal, Biología Vegetal y Ecología. Paraje Las Lagunillas s/n. 23071. Jaén, España. 5Mediterranea University of Reggio Calabria, Department “Agraria”. Località Feo di Vito, 89122. Reggio Calabria, Italy. *Corresponding author: [email protected], tel.: +351 968 979 085 Abstract Introduction: Forest and pre-forest fringe communities in the southwest of the Iberian Peninsula are semi-shaded perennial herbs of external fringe and open areas of evergreen or semi- deciduous woodlands and their pre-forestry mantles, linked to the Stachyo lusitanicae-Cheirolophenion sempervirentis suballiance. Objective: To evaluate the chorology, ecological features and floristic circumscription of the forest fringe communities of the southwestern Iberian Peninsula. Materials and methods: Forest fringe communities adscribed to the Stachyo lusitanicae- Cheirolophenion sempervirentis suballiance were analysed, using phytosociological approach (Braun- Blanquet methodology) and numerical analysis (hierarchical cluster analysis). -
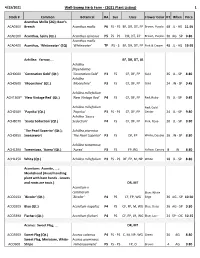
WSHF Catalog
4/23/2021 Well-Sweep Herb Farm - (2021 Plant Listing) 1 Stock # Common Botanical HA Sun Uses Flower ColorHT When Price Acanthus Mollis (2Q); Bear's ACA030X Breech Acanthus mollis P6 FS - PS BF, DR, DT, FP Brown, Purple- 48 JL - AG 11.95 ACA010X Acanthus, Spiny (Qt.) Acanthus spinosus P5 FS - PS DR, DT, FP Brown, Purple- 30 AG - SP 9.80 Acanthus mollis ACA040X Acanthus, `Whitewater' (2Q) `Whitewater' TP PS - S BF, DR, DT, FP Pink & Cream 48 JL - AG 19.95 Achillea: Yarrow, ... BF, DR, DT, LB Achillea filipendulina ACH000X `Coronation Gold' (Qt.) `Coronation Gold' P3 FS CF, DF, FP Gold 36 JL - SP 8.80 Achillea ACH050X `Moonshine' (Qt.) `Moonshine' P3 FS CF, DF, FP Gold 24 JN - SP 9.45 Achillea millefolium ACH130X* `New Vintage Red' (Qt.) `New Vintage Red' P4 FS CF, DF, FP Red, Ruby- 15 JL - SP 9.45 Achillea millefolium Red; Gold ACH250X `Paprika' (Qt.) `Paprika' P3 FS - PS CF, DF, FP Center 24 JL - SP 9.80 Achillea `Saucy ACH807X `Saucy Seduction' (Qt.) Seduction' P4 FS CF, DF, FP Pink, Rose- 20 JL - SP 9.80 `The Pearl Superior' (Qt.); Achillea ptarmica ACH095X Sneezewort `The Pearl Superior' P3 FS DF, FP White; Double 16 JN - SP 8.80 Achillea tomentosa ACH120X Tomentosa, `Aurea' (Qt.) `Aurea' P3 FS FP, RG Yellow, Canary- 8 JN 8.80 ACH125X White (Qt.) Achillea millefolium P3 FS - PS DF, FP, M, NP White 18 JL - SP 8.80 Aconitum: Aconite, ... ; Monkshood (Avoid handling plant with bare hands - Leaves and roots are toxic.) DR, MT Aconitum x cammarum Blue; White ACO015X `Bicolor' (Qt.) `Bicolor' P4 PS CF, FP, WG Edge 36 AG - SP 10.50 ACO020X Blue (Qt.) Aconitum napellus P4 PS CF, FP, M, WG Blue, Deep- 36 AG - SP 9.80 ACO339X Fischeri (Qt.) Aconitum fischeri P4 PS CF, FP, LB, WG Blue, Lav.- 24 SP - OC 10.15 Acorus: Sweet Flag, .. -

Chemical Compositions of the Volatile Oils and Antibacterial Screening of Solvent Extract from Downy Lavender
foods Article Chemical Compositions of the Volatile Oils and Antibacterial Screening of Solvent Extract from Downy Lavender 1, 1, 1 1 1 Chang Ha Park y, Ye Eun Park y, Hyeon Ji Yeo , Se Won Chun , Thanislas Bastin Baskar , Soon Sung Lim 2 and Sang Un Park 1,* 1 Department of Crop Science, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon 34134, Korea; [email protected] (C.H.P.); [email protected] (Y.E.P.); [email protected] (H.J.Y.); [email protected] (S.W.C.); [email protected] (T.B.B.) 2 Department of Food and Nutrition and Institute of Natural Medicine, Hallym University, Chuncheon 200-702, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-42-821-5730; Fax: +82-42-822-2631 These authors contributed equally to this work. y Received: 1 March 2019; Accepted: 16 April 2019; Published: 19 April 2019 Abstract: The discovery of a new species exhibiting more effective antibacterial properties is necessary because of the demand on Lavandula species, which continues to increase in a variety of industries. Lavandula pubescens might be a good alternative, as it exhibits strong antibacterial activity. In this study, the chemical composition of the essential oils from different organs (flowers, leaves, stems, and roots) of L. pubescens was identified using gas chromatography-mass spectrometry. Furthermore, the antimicrobial activities of different solvent extracts (methanol, ethanol, diethyl ether, hexane, and ethyl acetate) and different organ (flower, leaf, stem, and root) extracts of L. pubescens were evaluated. Only the ethyl acetate extracts of L. -

Histochemical and Phytochemical Analysis of Lamium Album Subsp
molecules Article Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids Agata Konarska 1, Elzbieta˙ Weryszko-Chmielewska 1, Anna Matysik-Wo´zniak 2 , Aneta Sulborska 1,*, Beata Polak 3 , Marta Dmitruk 1,*, Krystyna Piotrowska-Weryszko 1, Beata Stefa ´nczyk 3 and Robert Rejdak 2 1 Department of Botany and Plant Physiology, University of Life Sciences, Akademicka 15, 20-950 Lublin, Poland; [email protected] (A.K.); [email protected] (E.W.-C.); [email protected] (K.P.-W.) 2 Department of General Ophthalmology, Medical University of Lublin, Chmielna 1, 20-079 Lublin, Poland; [email protected] (A.M.-W.); [email protected] (R.R.) 3 Department of Physical Chemistry, Medical University of Lublin, Chod´zki4A, 20-093 Lublin, Poland; [email protected] (B.P.); offi[email protected] (B.S.) * Correspondence: [email protected] (A.S.); [email protected] (M.D.); Tel.: +48-81-445-65-79 (A.S.); +48-81-445-68-13 (M.D.) Abstract: The aim of this study was to conduct a histochemical analysis to localize lipids, terpenes, essential oil, and iridoids in the trichomes of the L. album subsp. album corolla. Morphometric examinations of individual trichome types were performed. Light and scanning electron microscopy Citation: Konarska, A.; techniques were used to show the micromorphology and localization of lipophilic compounds and Weryszko-Chmielewska, E.; iridoids in secretory trichomes with the use of histochemical tests. Additionally, the content of Matysik-Wo´zniak,A.; Sulborska, A.; essential oil and its components were determined using gas chromatography-mass spectrometry Polak, B.; Dmitruk, M.; (GC-MS). -

Achillea Millefolium L. جداسازی ژنهای لینالول سنتاز و پینن سنتاز از گیاه دارویی بومادران ) (
پژوهشهای ژنتیک گیاهی / جلد 2 / شماره 1 Achillea millefolium L. جداسازی ژنهای لینالول سنتاز و پینن سنتاز از گیاه دارویی بومادران ) ( مریم جاودان اصل1، حمید رجبی معماری2،*، داریوش نباتی احمدی2 و افراسیاب راهنما قهفرخی2 1- دانشآموخته کارشناسی ارشد، گروه زراعت و اصﻻحنباتات، دانشکده کشاورزی، دانشگاه شهید چمران، اهواز 3- استادیار، گروه زراعت و اصﻻحنباتات، دانشکده کشاورزی، دانشگاه شهید چمران، اهواز )تاریخ دریافت: 12/70/1232 – تاریخ پذیرش: 1232/13/30( چکیده بومادران ).Achillea millefolium L( گیاهی علفی و چندساله از خانوادهی گل ستتاره ایهتا )Asteraceae( متی باشتد استان بومادران دارای ترکیبهایی از جمله مونوترپن و سزکوئیترپنهای مختلف است که لینالول و پیتنن از اجتزای اصتلی تشتکیل دهنده آن میباشند این دو ترکیب دارای ارزش دارویی و اثرات ضدآفت و ضدمیکروبی هستند و در صنایع غذایی، عطرسازی و آرایشی و بهداشتی کاربرد دارند هدف از تحقیق حاضر، استفاده از راهکار آغازگرهای هرز برای جداسازی ژن های لینتالول سنتاز و پینن سنتاز از گیاه دارویی بومادران میباشد در این تحقیق RNA کل از گیاه بومادران استتخرا شتد، ستس ژنهتای موردنظر با استفاده از آغازگرهای هرز و واکتنش زنجیت رهای پلیمتراز )PCR( تکثیتر گردیدنتد نتتای حاصتل از PCR، تکثیتر باندهای مورد نظر به ترتیب حدود 037 و 357 جفت باز را نشان داد با آنالیزهای بیوانفورماتیکی، نتای توالییابی با دادههتای موجود در بانک ژن جهانی )NCBI( مقایسه گردید نتای بررسی تنوع بین گونهها و خت انوادههتای مختلتف گیتاهی و روابت فیلوژنتیکی بر اساس ژنهای Pis و Lis نشان داد بیشترین میزان مشابهت بین گیاه بومادران و درمنه )Artemisia annua( و نیز بین خانواده های Asteraceae و Lamiaceae وجود دارد، به نحوی که در یک گروه قرار گرفتند نتای این پتووهش، مشتابهت نسبتاً باﻻی توالی این ژنها را با ژنهای متناظر در سایر گیاهان نشان داد و صحت توالییابی را تأیید نمود واژگان کلیدی: آغازگر هرز، بومادران، پینن سنتاز، ترپنها، لینالول سنتاز Downloaded from pgr.lu.ac.ir at 4:08 IRST on Tuesday October 5th 2021 [ DOI: 10.29252/pgr.2.1.23 ] * نویسنده مسئول، آدرس پست الکترونیکی: [email protected] 32 …. -

Ofcanada Part13
THE INSECTS ANDARAOHNIDS OFCANADA PART13 The ofca,.m'ffitrslP; Coleo r* SgHHy'" THE INSECTS ANDARACHNIDS OFCANADA t%RT13 The Carrion Beetles of Canada and Alaska Coleoptera Silphidae and Agyrtidae Robert S. Andersonl and Stewart B. Peck2 Biosystematics Research Institute Ottawa, Ontario Research Branch Agriculture Canada Publication 1778 1985 rUniyersity of Alberta, Edmonton, Alberta 2Carleton University, Ottawa, Ontario oMinister of Supply and Services Canada 1985 Available in Canada through Authorized Bookstore Agents and other bookstores or by mail from Canadian Government Publishing Centre Supply and Services Canada Ottawa, Canada KIA 0S9 Catalogue No. A42-42,21985-l3E Canada: $7.00 ISBN 0-662-11752-5 Other Countries: $8.40 Price subject to change without notice Canadian Cataloguing in Publication Data Anderson, Robert Samuel The carrion beetles of Canada and Alaska (Coleoptera: Silphidae and Agyrtidae) (The Insects and arachnids of Canada, ISSN 0706-7313 ; pt. 13) (Publication ;1778) Includes bibliographical references and index. l. Silphidae. 2. Beetles - Canada. 3. Beetles -- Alaska. I. Peck, Stewart B. II. Canada. Agricul- ture Canada. Research Branch. III. Title. IV. Series. V. Series: Publication (Canada. Agri- culture Canada). English ; 1778. QL596.S5A5 1985 595.76 C85-097200-0 The Insects and Arachnids of Canada Part l. Collecting, Preparing, and Preserving Insects, Mites, and Spiders, compiled by J. E. H. Martin, Biosystematics Research Institute, Ottawa, 1977. 182 p. Price: Canada $3.50, other countries $4.20 (Canadian funds). Cat. No. A42-42/1977 -1. Partie 1. R6colte, prdparation et conservation des Insectes, des Acariens et des Araign6es, compil6 par J.E.H. Martin, Institut de recherches biosyst6- matiques, Ottawa, 1983. -

Essential Oils of Lamiaceae Family Plants As Antifungals
biomolecules Review Essential Oils of Lamiaceae Family Plants as Antifungals Tomasz M. Karpi ´nski Department of Medical Microbiology, Pozna´nUniversity of Medical Sciences, Wieniawskiego 3, 61-712 Pozna´n,Poland; [email protected] or [email protected]; Tel.: +48-61-854-61-38 Received: 3 December 2019; Accepted: 6 January 2020; Published: 7 January 2020 Abstract: The incidence of fungal infections has been steadily increasing in recent years. Systemic mycoses are characterized by the highest mortality. At the same time, the frequency of infections caused by drug-resistant strains and new pathogens e.g., Candida auris increases. An alternative to medicines may be essential oils, which can have a broad antimicrobial spectrum. Rich in the essential oils are plants from the Lamiaceae family. In this review are presented antifungal activities of essential oils from 72 Lamiaceae plants. More than half of these have good activity (minimum inhibitory concentrations (MICs) < 1000 µg/mL) against fungi. The best activity (MICs < 100) have essential oils from some species of the genera Clinopodium, Lavandula, Mentha, Thymbra, and Thymus. In some cases were observed significant discrepancies between different studies. In the review are also shown the most important compounds of described essential oils. To the chemical components most commonly found as the main ingredients include β-caryophyllene (41 plants), linalool (27 plants), limonene (26), β-pinene (25), 1,8-cineole (22), carvacrol (21), α-pinene (21), p-cymene (20), γ-terpinene (20), and thymol (20). Keywords: Labiatae; fungi; Aspergillus; Cryptococcus; Penicillium; dermatophytes; β-caryophyllene; sesquiterpene; monoterpenes; minimal inhibitory concentration (MIC) 1. Introduction Fungal infections belong to the most often diseases of humans. -

Lavandula Luisieri and Lavandula Viridis Essential Oils As Upcoming Anti-Protozoal Agents: a Key Focus on Leishmaniasis
applied sciences Article Lavandula Luisieri and Lavandula Viridis Essential Oils as Upcoming Anti-Protozoal Agents: A Key Focus on Leishmaniasis Marisa Machado 1,2 , Natália Martins 3,4,* ,Lígia Salgueiro 5,6, Carlos Cavaleiro 5,6 and Maria C. Sousa 5,7 1 Instituto de Investigação e Formação Avançada em Ciências e Tecnologias da Saúde (CESPU), Rua Central de Gandra, 1317, 4585-116 Gandra PRD, Portugal 2 Centro de Investigação em Biodiversidade e Recursos Genéticos (CIBIO-UP), Universidade do Porto, 4485-661 Vairão, Portugal 3 Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal 4 Institute for Research and Innovation in Health (I3S), University of Porto, 4200-135 Porto, Portugal 5 Faculty of Pharmacy, University of Coimbra, 3000-548 Coimbra, Portugal 6 Chemical Process Engineering and Forest Products Research Center, University of Coimbra, 3030-790 Coimbra, Portugal 7 Center for Neurosciences and Cell Biology (CNC), University of Coimbra, 3030-790 Coimbra, Portugal * Correspondence: [email protected] Received: 5 June 2019; Accepted: 12 July 2019; Published: 29 July 2019 Abstract: Background and objectives: Leishmania species is the causative agent of leishmaniasis, a broad-spectrum clinical condition that can even be life-threatening when neglected. Current therapeutic strategies, despite beings highly cost-effective, have been increasingly associated with the appearance of drug-resistant microorganisms. Thus, an increasing number of thorough studies are needed towards upcoming drug discovery. This study aims to reveal the anti-protozoa activity of Lavandula luisieri and Lavandula viridis essential oils (EO) and their main components (1,8-cineole, linalool, and borneol). Materials and Methods: L. luisieri and L. -

Quinto-Canas 2018 Plant Soc Agrostion Editor.Pdf
Plant Sociology, Vol. 55, No. 1, June 2018, pp. 21-29 DOI 10.7338/pls2018551/02 The Agrostion castellanae Rivas Goday 1957 corr. Rivas Goday & Rivas- Martínez 1963 alliance in the southwestern Iberian Peninsula R. Quinto-Canas1,2, P. Mendes3, C. Meireles3, C. Mussarella4, C. Pinto-Gomes3 1Faculty of Sciences and Technology, University of Algarve, Campus de Gambelas, P-8005-139 Faro, Portugal. 2CCMAR – Centre of Marine Sciences (CCMAR), University of Algarve, Campus de Gambelas, P-8005-139 Faro, Portugal. 3Department of Landscape, Environment and Planning; Institute of Mediterranean Agricultural and Environmental Sciences (ICAAM), School of Science and Technology, University of Évora, Rua Romão Ramalho 59, P-7000-671 Évora, Portugal. 4Department of Agraria, “Mediterranea” University of Reggio Calabria, Località Feo di Vito, I-89122 Reggio Cala- bria, Italy. Abstract The water courses of southern Portugal are ecosystems subject to constant fluctuations between periods of flooding and desiccation associated with seasonal dryness. In these unstable ecological conditions, a considerable diversity of riparian plant communities occurs. The objective of this study, carried out in the Monchique Sierran and Andévalo Districts, is to compare the perennial grasslands community dominated by Festuca ampla Hack, using a phytosociological approach (Braun-Blanquet methodology) and numerical analysis (hierarchical cluster analysis and ordination). From these results, a new hygrophilous community of perennial grasslands type was identified, Narcisso jonquillae-Festucetum amplae, as a result of the floristic, ecological and biogeographical differences from other associations already described within the Agrostion castellanae alliance, in the southwestern Iberian Peninsula. The association occurs in the thermomediterranean to mesomediterranean belts under dry to sub-humid ombrotypes, on siliceous soils that have temporary waterlogging. -

Key to the Carrion Beetles (Silphidae) of Colorado & Neighboring States
Key to the carrion beetles (Silphidae) of Colorado & neighboring states Emily Monk, Kevin Hinson, Tim Szewczyk, Holly D’Oench, and Christy M. McCain UCB 265, Department of Ecology & Evolutionary Biology, and CU Museum of Natural History, Boulder, CO 80309, [email protected], [email protected] Version 1 posted online: March 2016 This key is based on several identification sources, including Anderson & Peck 1985, De Jong 2011, Hanley & Cuthrell 2008, Peck & Kaulbars 1997, Peck & Miller 1993, and Ratcliffe 1996. We include all species known from Colorado and those in the surrounding states that might occur in Colorado. Of course, new species may be detected, so make sure to investigate unique individuals carefully. We have included pictures of each species from specimens of the Entomology collection at the CU Museum of Natural History (UCM), the Colorado State C.P. Gillette Museum of Arthropod Diversity (GMAD), and the Florida State Collection of Arthropods (FSCA). A glossary of terms, a list of the states where each species has been detected, and references can be found after the key. We would appreciate reports of omitted species or species from new localities not stated herein. First step—ID as a silphid: Large size, body shape, and antennal club are usually distinctive. Body usually 10-35 mm, moderately to strongly flattened. Elytra broad toward rear, either loosely covering abdomen or short, exposing 1-3 segments. Antennae often ending in a hairy, three-segmented club, usually preceded by two or three enlarged but glabrous segments (subfamily Silphinae) or antennomeres 9-11 lammellate (subfamily Nicrophorinae). Black, often with red, yellow, or orange markings.