A Statistical Assessment of the Status and Trends of Antarctic and Subantarctic Seabirds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Birdlife Australia Rarities Committee Unusual Record Report Form
BirdLife Australia Rarities Committee Unusual Record Report Form This form is intended to aid observers in the preparation of a submission for a major rarity in Australia. (It is not a mandatory requirement) Please complete all sections ensuring that you attach all relevant information including any digital images (email to [email protected] or [email protected]). Submissions to BARC should be submitted electronically wherever possible. Full Name: Rob Morris Office Use Address: Phone No: Robert P. Morris, Email: Full Name: Andrew Sutherland (first noticed the second bird) Address: Phone No: Email: Species Name: Broad-billed Prion Scientific Name: Pachyptila vittata Date(s) and time(s) of observation: 11 August 2019 First individual photographed at 12.22 – last bird photographed at 13.11. How long did you watch the bird(s)? c30+ minutes – multiple sightings of 2 birds (possibly 3) and then an additional sighting of 1 bird 20 minutes later whilst travelling, flying past and photographed. First and last date of occurrence: 11 August 2019 Distance to bird: Down to approximately 20-30 m Site Location: SE Tasmania. Approximately 42°50'36.30"S 148°24'46.23"E 22NM ENE of Pirates Bay, Eaglehawk Neck. We went north in an attempt to seek lighter winds and less swell and avoid heading straight into the strong SE winds and southerly swell. Habitat (describe habitat in which the bird was seen): Continental slope waters at a depth of approximately 260 fathoms. Sighting conditions (weather, visibility, light conditions etc.): Weather: Both days were mostly cloudy with occasional periods of bright sunshine. -

DIET and ASPECTS of FAIRY PRIONS BREEDING at SOUTH GEORGIA by P.A
DIET AND ASPECTS OF FAIRY PRIONS BREEDING AT SOUTH GEORGIA By P.A. PRINCE AND P.G. COPESTAKE ABSTRACT A subantarctic population of the Fairy Prion (Pachyprzla turtur) was studied at South Georgia in 1982-83. Full measurements of breeding birds are given, together with details of breeding habitat, the timing of the main breeding cycle events, and chick growth (weight and wing, culmen and tarsus length). Regurgitated food samples showed the diet to be mainly Crustacea (96% by weight), fish and squid comprising the rest. Of crustaceans, Antarctic krill made up 38% of items and 80% by weight. Copepods (four species, mostly Rhincalanus gigas) made up 39% of items but only 4% by weight; amphipods [three species, principally Themisto gaudichaudii made up 22% of items and 16% by weight. Diet and frequency of chick feeding are compared with those of Antarctic Prions and Blue Petrels at the same site; Fairy Prions are essentially intermediate. INTRODUCTION The Fairy Prion (Pachyptila turtur) is one of six members of a genus confined to the temperate and subantarctic regions of the Southern Hemisphere. With the Fulmar Prion (P. crassirostris), it forms the subgenus Pseudoprion. Its main area of breeding distribution is between the Antarctic Polar Front and the Subtropical Convergence. It is widespread in the New Zealand region, from the north of the North Island south to the Antipodes Islands and Macquarie Island, where only about 40 pairs survive (Brothers 1984). Although widespread in the Indian Ocean at the Prince Edward, Crozet and Kerguelen Islands, in the South Atlantic Ocean it is known to breed only on Beauchene Island (Falkland Islands) (Strange 1968, Smith & Prince 1985) and South Georgia (Prince & Croxall 1983). -

UNEP/CBD/RW/EBSA/SIO/1/4 26 June 2013
CBD Distr. GENERAL UNEP/CBD/RW/EBSA/SIO/1/4 26 June 2013 ORIGINAL: ENGLISH SOUTHERN INDIAN OCEAN REGIONAL WORKSHOP TO FACILITATE THE DESCRIPTION OF ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS Flic en Flac, Mauritius, 31 July to 3 August 2012 REPORT OF THE SOUTHERN INDIAN OCEAN REGIONAL WORKSHOP TO FACILITATE THE DESCRIPTION OF ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS1 INTRODUCTION 1. In paragraph 36 of decision X/29, the Conference of the Parties to the Convention on Biological Diversity (COP 10) requested the Executive Secretary to work with Parties and other Governments as well as competent organizations and regional initiatives, such as the Food and Agriculture Organization of the United Nations (FAO), regional seas conventions and action plans, and, where appropriate, regional fisheries management organizations (RFMOs), with regard to fisheries management, to organize, including the setting of terms of reference, a series of regional workshops, with a primary objective to facilitate the description of ecologically or biologically significant marine areas (EBSAs) through the application of scientific criteria in annex I of decision IX/20, and other relevant compatible and complementary nationally and intergovernmentally agreed scientific criteria, as well as the scientific guidance on the identification of marine areas beyond national jurisdiction, which meet the scientific criteria in annex I to decision IX/20. 2. In the same decision (paragraph 41), the Conference of the Parties requested that the Executive Secretary make available the scientific and technical data and information and results collated through the workshops referred to above to participating Parties, other Governments, intergovernmental agencies and the Subsidiary Body on Scientific, Technical and Technological Advice (SBSTTA) for their use according to their competencies. -

Spatial Segregation of Prions and Blue Petrels Is Explained by Differences in Preferred Sea Surface Temper
Animal behaviour Cool, cold or colder? Spatial segregation rsbl.royalsocietypublishing.org of prions and blue petrels is explained by differences in preferred sea surface temperatures Research 1 2 2 2 Cite this article: Quillfeldt P, Cherel Y, Delord Petra Quillfeldt , Yves Cherel , Karine Delord and Henri Weimerkirch K, Weimerkirch H. 2015 Cool, cold or colder? 1Department of Animal Ecology and Systematics, Justus-Liebig-Universita¨t Giessen, 35392 Giessen, Germany Spatial segregation of prions and blue petrels 2Centre d’Etudes Biologiques de Chize´, UMR 7372 CNRS-Universite´ de La Rochelle, 79360 Villiers-en-Bois, France is explained by differences in preferred sea surface temperatures. Biol. Lett. 11: 20141090. The Southern Ocean provides one of the largest environmental gradients on http://dx.doi.org/10.1098/rsbl.2014.1090 Earth that lacks geographical barriers, and small but highly mobile petrels living there may offer fine models of evolution of diversity along environmental gradients. Using geolocation devices, we investigated the winter distribution of closely related petrel species breeding sympatrically in the southern Indian Received: 31 December 2014 Ocean, and applied ecological niche models to compare environmental con- ditions in the habitat used. We show that thin-billed prions (Pachyptila Accepted: 27 March 2015 belcheri), Antarctic prions (Pachyptila desolata) and blue petrels (Halobaena caerulea) from the Kerguelen archipelago in the southern Indian Ocean segre- gate latitudinally, sea surface temperature being the most important variable separating the distribution of the species. Antarctic prions spent the winter Subject Areas: north of the Polar Front in temperate waters, whereas blue petrels were ecology, behaviour found south of the Polar Front in Antarctic waters. -

AC10 Doc 11 Rev 1 Agenda Item 11.1
AC10 Doc 11 Rev 1 Agenda Item 11.1 Tenth Meeting of the Advisory Committee Wellington, New Zealand, 11 – 15 September 2017 Report of the Population and Conservation Status Working Group Population and Conservation Status Working Group CONTENTS 1. WELCOME AND OPENING REMARKS ......................................................................................... 3 2. MEMBERSHIP AND INTRODUCTION............................................................................................ 3 3. ADOPTION OF THE AGENDA ........................................................................................................ 3 4. PROGRESS REPORTS ................................................................................................................... 3 4.1. Database updates ............................................................................................................................. 3 4.2. Updates and Reviews of ACAP Species Assessments .................................................................... 4 4.3. List of researchers with access to tissues from bycaught birds ........................................................ 4 5. POPULATION STATUS AND TRENDS .......................................................................................... 4 5.1. Current population trends of ACAP species ..................................................................................... 4 6. THREATS AND PRIORITISATION ................................................................................................. 8 6.1. Updates -

Master Wildlife List-Peninsula
Antarctic Peninsula Expedition Wildlife List Date Family Species Twitcher List 8-Mar 9-Mar 10-Mar 11-Mar 12-Mar 13-Mar 14-Mar 15-Mar 16-Mar 17-Mar Adelie X X Chinstrap X X X X X X Emperor Penguins Gentoo X X X X X X X Macaroni X X Magellanic X X Unidentifed X X X Black-browed X X X X X Grey-headed X X X X Light-mantled Sooty X X Albatross Northern Royal X X X Southern Royal X X X Wandering X X X Northern X X X X Southern X X X X X X X Giant Petrel Southern (white morph) X X X Unidentified Soft-plumaged Petrel X X X X Gadfly Petrels Soft-plumaged Petrel (Dark) X X Antarctic Petrel Cape Petrel X X X X X X Snow Petrel ? ? Petrels Southern Fulmar X X X X White-chinned Petrel X X X Grey Petrel Great Shearwater X X Shearwaters Sooty Shearwater X X X X Blue Petrel Antarctic Prion X X X X BIRDS Blue Petrels & Prions Fairy Prion Slender-billed Prion X X Unidentified Black-bellied X X X X Grey-backed Storm-petrels Wilson’s X X X X X X X X Unidentified Common Diving-petrels Magellanic Unidentified X X X Antarctic Shag X X X X X X Shags Imperial Shag X X Rock Shag X X Chilean Skua X X South Polar Skua X X X X X X Skuas Subantarctic Skua X X X X X X Unidentified X X Brown-hooded Gull X X Gulls Dolphin Gull X X Kelp Gull X X X X X X X Antarctic X X X X X Terns Arctic South American Blk-crowned Night Heron Blackish Oystercatcher Shorebirds Magellanic Oystercatcher Magellanic Snipe Pale-faced Sheathbill X X X X Other Arnoux's Beaked Blue Cuvier's Beaked Fin Whale Hector's Beaked Humpback X X X Long-finned Pilot Whales Minke X X X X Sei X X X X Southern Bottlenose Southern Right Sperm Beaked (sp.) X X MAMMALS Unidentified Dusky Hourglass X X Orca (Killer Whale) X X Dolphins Peale’s X X Other Unidentified Crabeater X X X X X Leopard X X X X Phocids Southern Elephant X X X Weddell X X X X Antarctic Fur Seal X X X X X Otariids South American Fur Seal South American Sealion OTHER Chilean swallow X X Salp X 8-Mar 9-Mar 10-Mar 11-Mar 12-Mar 13-Mar 14-Mar 15-Mar 16-Mar 17-Mar Twitcher List Date www.oneoceanexpedi,ons.com. -

Arctocephalus Gazella, at Iles Kerguelen
Polar Biol (2002) 25: 269–279 DOI 10.1007/s00300-001-0339-6 ORIGINAL PAPER Mary-Anne Lea Æ Mark Hindell Æ Christophe Guinet Simon Goldsworthy Variability in the diving activity of Antarctic fur seals, Arctocephalus gazella, at Iles Kerguelen Accepted: 15 October 2001 / Published online: 28 November 2001 Ó Springer-Verlag 2001 Abstract Intra-population variation in diving behaviour Introduction of lactating Antarctic fur seals (Arctocephalus gazella) was studied at the Kerguelen Archipelago (49°07’S, The diving behaviour of marine predators, quantified by 70°45’E) during the austral summers of 1998–2000. Dive the use of time-depth recorders (TDRs), is one of the data were successfully recorded for 112 seals equipped most commonly used measures for differentiating for- with time-depth recorders during 117 foraging trips. All aging activity between individuals and populations, both seals displayed bouts of diving activity and the nocturnal spatially and temporally (Antonelis et al. 1990; Hindell foraging behaviour typical of otariids preying on pelagic et al. 1991; Schreer and Testa 1996; Bowen et al. 1999; fish and squid. Mean dive depth (53 m) was consider- Cherel et al. 1999; Bonadonna et al. 2000; Georges et al. ably deeper than recorded for this species at other sites. 2000a, b). In recent years the diving behaviour of several Four diving behaviour groups were identified: (1) deep marine predators has also been linked to spatial and divers (n=60); (2) shallow-active divers (n=45); (3) temporal variability in the availability of prey species shallow divers (n=9); (4) daytime divers (n=3). The (Boyd et al. -

ACAP Colony Database Analysis
AC5 Doc 33 Agenda item 8.3 Agreement on the Conservation of Albatrosses and Petrels Fifth Meeting of Advisory Committee Mar del Plata, Argentina, 13 – 17 April 2010 ________________________________________________________________ ACAP Internationally Important Breeding Areas Author: BirdLife International „This paper is presented for consideration by ACAP and may contain unpublished data, analyses, and/or conclusions subject to change. Data in this paper shall not be cited or used for purposes other than the work of the ACAP Secretariat, ACAP Advisory Committee or their subsidiary Working Groups without the permission of the original data holders.‟ AC5 Doc 33 Agenda item 8.3 ACAP Internationally Important Breeding Sites: IBA analysis of the ACAP colony database Paper submitted to AC5 Uthgra Sasso Hotel, Mar del Plata, Argentina 13-17 April 2010 BirdLife International Feb 2010 AC5 Doc 33 Agenda item 8.3 Recommended citation: BirdLife International (2010) ACAP Internationally Important Breeding Sites: IBA analysis of the ACAP colony database. Cambridge, UK: BirdLife International. Compiled by B. Lascelles, BirdLife International Secretariat and BirdLife Global Seabird Programme, Cambridge, UK Acknowledgements Thanks to Richard Phillips and Wieslawa Misiak for assistance in extracting the relevant information from the ACAP colony database, and to Lincoln Fishpool for comments on the draft of this document. Abstract This paper provides information on the breeding sites for ACAP-listed species that are known to hold ≥1% of the global population of the species in question. Information on the breeding site locations and populations present at each site were taken from the ACAP colony database. Information on the global populations of ACAP-listed species was taken from the BirdLife World Bird Database. -

Densities of Antarctic Seabirds at Sea and the Presence of the Krill Eupha Usia Superba
DENSITIES OF ANTARCTIC SEABIRDS AT SEA AND THE PRESENCE OF THE KRILL EUPHA USIA SUPERBA BRYAN S. OBST Departmentof Biology,University of California,Los Angeles, California 90024 USA ABSTRACT.--Theantarctic krill Euphausiasuperba forms abundant,well-organized schools in the watersoff the AntarcticPeninsula. Mean avian densityis 2.6 timesgreater in waters where krill schoolsare present than in waters without krill schools.Seabird density is a good predictorof the presenceof krill. Seabirddensity did not correlatewith krill density or krill schooldepth. Disoriented krill routinely were observedswimming near the surface above submergedschools, providing potential prey for surface-feedingbirds. Responsesof seabird speciesto the distribution of krill schoolsvaried. The small to me- dium-sizeprocellariiform species were the best indicatorsof krill schools;large procellari- iforms and coastalspecies were poor indicators.Pygoscelis penguins occurredat high den- sitiesonly in the presenceof krill schools.These responses are consistentwith the constraints imposedby the metabolicrequirements and reproductivestrategies of eachof thesegroups. Krill schoolswere detectednear the seasurface throughout the day. Correlationsbetween seabirddensity and the presenceof krill during daylight hourssuggest that diurnal foraging is important to the seabirdsof this region. Received19 December1983, accepted4 December 1984. RELATIVELY little is known about the factors birds depend on directly or indirectly for food influencing the distribution of seabirdsin the (Haury et al. 1978). These observationssuggest marine habitat. The past decade has produced that relatively small-scalephenomena, such as a number of studiesattempting to correlatepat- local concentrationsof prey, may be of major terns of avian abundance and distribution with importance in determining the patterns of sea- physical featuresof the oceansuch as currents bird distribution within the broad limits set by and convergences,water masses,and temper- featuresof the physical ocean. -

Population Trends of Penguins in the French Southern Territories
Author's personal copy Polar Biology (2020) 43:835–850 https://doi.org/10.1007/s00300-020-02691-6 ORIGINAL PAPER Population trends of penguins in the French Southern Territories Christophe Barbraud1 · Karine Delord1 · Charles A. Bost1 · Adrien Chaigne2 · Cédric Marteau2 · Henri Weimerskirch1 Received: 31 July 2019 / Revised: 21 April 2020 / Accepted: 29 May 2020 / Published online: 3 June 2020 © Springer-Verlag GmbH Germany, part of Springer Nature 2020 Abstract Penguins are important top consumers in marine food webs and are one of the most threatened bird families, especially by climate change and food web alterations by marine fsheries. Yet, long-term population trends are lacking or are uncertain for many populations. Seven species of penguins breeding at the French Southern Territories in the southern Indian Ocean on the Crozet, Kerguelen, Saint-Paul–Amsterdam archipelagos and in Terre Adélie/Adelie Land, Antarctica are monitored regularly. This monitoring started in the early 1950s and most populations have been surveyed during the past four years, allowing assessments of population trends. King penguins increased at nearly all breeding sites within the Crozet and Ker- guelen archipelagos. Emperor penguins have decreased at Terre Adélie/Adelie Land, with a partial recovery of the colony during the 2010s. Gentoo penguin populations at Crozet and Kerguelen are highly variable but stable. Adélie penguins have been increasing in Terre Adélie/Adelie Land. The trends in eastern rockhopper penguins vary between colonies and archipelagos. Northern rockhopper penguins have continuously decreased in numbers at Amsterdam Island, but appear to have increased at the nearby Saint-Paul Island. Macaroni penguins have frst increased and then stabilized since the 2000s at Kerguelen and are stable at the Crozet Islands. -
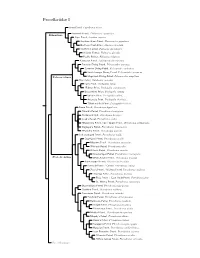
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Durham Research Online
Durham Research Online Deposited in DRO: 23 June 2014 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Hodgson, D.A. and Graham, A.G.C. and Roberts, S.J. and Bentley, M.J. and O¡ Cofaigh, C. and Verleyen, E. and Vyverman, W. and Jomelli, V. and Favier, V. and Brunstein, D. and Verfaillie, D. and Colhoun, E.A. and Saunders, K.M. and Selkirk, P.M. and Mackintosh, A. and Hedding, D.W. and Nel, W. and Hall, K. and McGlone, M.S. and Van der Putten, N. and Dickens, W.A. and Smith, J.A. (2014) 'Terrestrial and submarine evidence for the extent and timing of the Last Glacial Maximum and the onset of deglaciation on the maritime-Antarctic and sub-Antarctic islands.', Quaternary science reviews., 100 . pp. 137-158. Further information on publisher's website: http://dx.doi.org/10.1016/j.quascirev.2013.12.001 Publisher's copyright statement: c 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/). Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders.