The Comparative Study on Sorption of Basic Violet 10 by Cost-Effective Sorbents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assorted Variety of Genuine Mangrove and Their Partners in East Drift Locale
International Journal of Botany Studies International Journal of Botany Studies ISSN: 2455-541X Impact Factor: RJIF 5.12 www.botanyjournals.com Volume 3; Issue 5; September 2018; Page No. 20-24 Assorted variety of genuine mangrove and their partners in east drift locale of Pichavaram Tamil Nadu India and improve to change of mangrove plants, and its remedial properties with a mangrove knowledgebase Kumaravel S1, Vinoth R2, Ranganathan R3* 1-3 Division of mangrove physiology lab, Department of Botany, Annamalai University, Annamalai Nagar, Chidambaram, Tamil Nadu, India Abstract Mangroves are embraced with exceptional adjustment to outrageous conditions in tropical and subtropical areas of the world. It has a rich wellspring of auxiliary metabolites. The present investigation centres the assorted variety status examination of genuine mangroves and their partners in pichavaram and its environment. By guide field perception and examining to adjacent occupants. From the perception the decent variety level fluctuates starting with one place then onto the next, because of some natural factors, for example, atmospheres, tidal variables, shortage of learning about mangroves to the informed and town people groups and anthropogenic weights and so on it can instigate mangrove assorted variety harm and misfortune, decrease the human important items which is gotten from mangrove timberland. Must know the exact information from past examines, researchers, logical fields like morphology, life structures, physiology, Scientific classification, Biology, development stages, proliferation levels, and so on. Comprehend between the living space factors and propensity nature can help to dispersing by species required situations, it can normally incite self-insurance level high in plants itself. -

Annexure-District Survey Report
3/6/2017 Home TamilNadu Map Cuddalore District Profile Print CUDDALORE DISTRICT PROFILE • Cuddalore district is bounded by Villuppuram district in the north and northwest, Perambalur district in the southwest, and Ariyalur and Nagapattinam districts in the South and Bay of Bengal in the east. • Cuddalore district lies between 11º09'00’’N to 11º53'00’’ N Latitude, 78º52'00’’E to 79º51’00’’E Longitude and has an areal extent of 3706 sq.km • There are 13 Blocks, 683 Villages and 3639 Habitations in the District. Physiography and Drainage: • Cuddalore district being a coastal zone is mostly covered by plain terrain, without any high relief zone except some sedimentary high ground in Virudhachalam, Cuddalore and Panruti blocks. Rainfall: : Acutal Rainfall in mm Normal Rainfall in mm 2011 2012 2013 2014 2015 1461.4 883.20 977.7 1218.1 1705.7 1206.7 Geology: Rock Type Geological Formation Sandstone, Conglomerate, Sedimentary Rock 90% Gneiss, Charnockite, Marine Hard Rock 10 % deposits and Alluvium Hydrogeology: Type of aquifer Fairly thick but discontinuous confined to semi confined aquifers. Alluvium Tertiary Hard rock Aquifer parameters Well yield in lpm 150 Hard rock 60120 Transmissivity (T) m 2 /day 98 46134 16160 Permeability (K) m/day 19.7 1633 5 – 20 Sp. capacity. lpm/md 208 78.17 27224 Ground Water Level:: The Ground Water levels from the 42 number of observation wells of TWAD have been analysed for PostMonsoon and Pre Monsoon. Since 1991, average Ground water level in m Below Ground Level for pre and post monsoon is as follows: 1/3 3/6/2017 Sustainability:: With a view to enhance the Sustainability of the drinking water sources, recharge structures are being implemented by TWAD Board under various State and Central Government assistances. -

The Architectural and Cultural Heritage of Chidambaram Nataraja Temple - a Study
© 2019 JETIR June 2019, Volume 6, Issue 6 www.jetir.org (ISSN-2349-5162) THE ARCHITECTURAL AND CULTURAL HERITAGE OF CHIDAMBARAM NATARAJA TEMPLE - A STUDY Dr. P. Selvamani Assistant Professor Department of History Annamalai University, Annamalai Nagar – 608002. The celebrated Nataraja temple at Chidambaram or Tillai as it is otherwise called, of Cuddalore District in Tamil Nadu is too well known to all.1 Chidambaram, known from time immemorial as ‘Koyil’ (the temple) has also other names such as Sitrambalam, Puliyur, Chitrakoota, Perumbarapuliyur, Pundareekapuram.2 From the early beginning of the origin of the Nataraja temple at Chidambaram, the most famed of five southern temples dedicated to Siva, represent Him as the deity of five elements – Earth (Kanchipuram) Water (Thiruvannaikkal), Fire (Tiruvannamalai), Air (Kalahasthi) and Ether (Chidambaram). At Chidambaram, Siva is represented as the element of Ether (Akasha).3 Chidambaram is the centre of the Saivite cult. The temple complex covers a vast area and is located in the centre of the city. The name is derived from the most important portion of the Nataraja temple, called ‘Chit-Ambala’ meaning the ‘atmosphere of wisdom’, or Mystic Hall.4 This concedes within itself the secret symbol of God. When a veil over a semi-circular doorway in the ‘sanctum sanctorum’ is parted, it reveals more ‘ethereal space’ - symbolic of Siva in His manifestation of the “Formless Form” in “invisible presence” - a profound indication of a solution to the mystery of life, which is explained in one word - “Nothingness”.5 The temple is a perfect synthesis of two concepts - Siva as the cosmic dancer and Siva as “Akasha Linga” totally formless - “Chit-Ambaram” literally means the “Ether of Knowledge”. -

Survey of Mangroves and Their Associates in Pichavaram Mangrove Forest
R. Ranganathan et al., IJSIT, 2018, 7(2), 176-182 SURVEY OF MANGROVES AND THEIR ASSOCIATES IN PICHAVARAM MANGROVE FOREST S. Kumaravel and R. Ranganathan* Department of Botany, Annamalai University, Annamalai Nagar, Chidambaram, Tamil Nadu, India – 608 002. ABSTRACT Mangroves are the most productive coastal ecosystems in the world. The Pichavaram mangrove forest is rich mangrove diversity in Tamil Nadu. The present survey was conducted in the Pichavaram mangrove forest including Killai, Pichavaram and T.S. Pettai. Totally 25 species were recorded in the study period including 12 mangroves and 13 mangrove associated plants. In Mangroves, Avicennia and Rhizophora species were found to be dominant species in Pichavaram mangrove forest. Six dominant associate species were found such as Suaeda maritima, Suaeda monoica, Ipomoea pes-caprae and Sesuvium portulacastrum. Xylocarpus mekongensis is an endangered species and it was recorded in a very few. The information generated from this study will serve as a baseline to develop adaptive management strategies in anticipation of sea-level rise, setting of conservation priorities, monitor, deforestation and forest degradation, improve terrestrial carbon accounting and quantify the role of mangrove forests in saving lives and property from natural disasters such as tsunamis. Key words: Mangroves, Associates, Avicennia, Rhizophora, Conservation. 176 IJSIT (www.ijsit.com), Volume 7, Issue 2, March-April 2018 R. Ranganathan et al., IJSIT, 2018, 7(2), 176-182 INTRODUCTION Mangroves are among the most productive coastal ecosystems in the world (Kathiresan et al., 2001). They are confined to the tropics and subtropics, which dominate approximately 75% of the world’s coastline between 25° N and 25° S and are estimated to cover an area of 1.7 to 2.0× 105 km2 (Borges et al., 2003). -

The Emergence of British Imperialism on the Coromandel Coast Through
International Journal of Disaster Recovery and Business Continuity Vol.11, No. 3, (2020), pp. 2625–2629 The Emergence Of British Imperialism On The Coromandel Coast Through Defensive Architecture 1Mr .P.Arumugasamy, Ph.D Research Scholar, Department of History, Annamalai University, Annamalai Nagar-605 602 , 2Dr. Hameed Basha.B, Assistant professor, Department of History, Arignar Anna Government Arts College (Deputed from Annamalai University), Villupuram-605 608, Introduction European countries were fascinated over the spices which prevalent in India and its nearby islands. These regions has been renowned as ‘spice islands’ in south Asia, acted as the lucrative market in 16th and 17th century A.D. Yesteryear, the spice were traded via silk routes (land route) from Asia to Europe. In 1453 A.D, a trading center or hub was captured by the ottoman Turks which shutdown the Europeans trading activities. At the time, Europeans have using the spices for enormously would leads huge demand in Europe. Henceforth, European countries have tried to find a new route to reach India by their ships. On the historic path five Europeans countries, (especially geographically situated in Atlantic Ocean) like Portuguese, Dutch, Danish, British and French, have planned and proceed to identify the shortest route for India. In this battle English descended later while Portuguese, Dutch, and Danish constructed their prominent position there. However, British come almost last to fulfill the spice needs by its country. Once they came to India for trade but they get the great chance to emergence their kingdom in India. Finally, British dominated the entire world by its policies, administrative capabilities, and wise Governors. -

Private Schools Fee Determination Committee Chennai-600 006 - Fees Fixed for the Year 2013-2016 - District: Cuddalore Sl
PRIVATE SCHOOLS FEE DETERMINATION COMMITTEE CHENNAI-600 006 - FEES FIXED FOR THE YEAR 2013-2016 - DISTRICT: CUDDALORE SL. SCHOOL HEARING SCHOOL NAME & ADDRESS YEAR LKG UKG I II III IV V VI VII VIII IX X XI XII NO. CODE DATE 2013 - 14 9900 9900 11000 11000 11000 11000 11000 11400 11400 11400 12200 12200 - - KRISHNASAMY MAT SCHOOL OF EXCELLENCE 1 260001 S KUMARAPURAM 29-04-2013 2014 - 15 10890 10890 12100 12100 12100 12100 12100 12540 12540 12540 13420 13420 - - MARUTHADU ROAD CUDDALORE 2015 - 16 11979 11979 13310 13310 13310 13310 13310 13794 13794 13794 14762 14762 - - 2013 - 14 3500 3500 4000 4000 4000 4000 4000 4550 4550 4550 5700 5700 - - Sarva Seva Matriculation School 2 260002 Killai Town Panchayat, 18-03-2013 2014 - 15 3850 3850 4400 4400 4400 4400 4400 5005 5005 5005 6270 6270 - - M.G.R Nagar, Cuddalore - 608 102. 2015 - 16 4235 4235 4840 4840 4840 4840 4840 5506 5506 5506 6897 6897 - - 2013 - 14 9000 9000 11260 11260 11260 11260 11260 13000 13000 13000 14000 14000 - - St. Antony Matriculation School. 3 260004 Block - 29 29-4-2013 2014 - 15 9900 9900 12386 12386 12386 12386 12386 14300 14300 14300 15400 15400 - - Neyveli 607807 Cuddalore Dt. 2015 - 16 10890 10890 13625 13625 13625 13625 13625 15730 15730 15730 16940 16940 - - 2013 - 14 - - - - - - - 4570 4570 4570 - - - - O.P.R.HIGH SCHOOL 4 260006 THOLUDUR 18-03-2013 2014 - 15 - - - - - - - 5027 5027 5027 - - - - CUDDALORE 2015 - 16 - - - - - - - 5530 5530 5530 - - - - 2013 - 14 3800 3800 4800 4800 4800 4800 4800 - - - - - - - John Dewey Nursery & Primary School 5 260007 Memepalayam, 23-4-2013 2014 - 15 4180 4180 5280 5280 5280 5280 5280 - - - - - - - Panruti - 607106 Cuddalore Dt. -
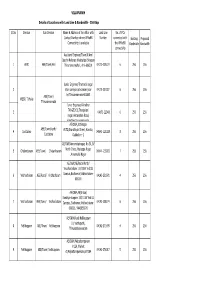
VILLUPURAM Sl.No Division Sub-Division Name & Address Of
VILLUPURAM Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity Assistant Engineer/Town/N/Arni Opp to Nellarasi Viyabarigal Snagam 1 ARNI AEE/Town/Arni Thirumana mahal , Arni-606301 04173-225224 6 256 256 Junior Engineer/Thamarai nagar 2 Visiri samiyar ashramam(near 04175-237387 6 256 256 AEE/Town/ by)Tiruvannamalai-606603 WEST/ T.Malai Thiruvannamalai Junior Engineer/Kilnathur 3 TANGEDCO,Thenpalani 04175-223481 6 256 256 nagar,Vettavalam Road, kilnathur,Tiruvannamalai. AE/O&M,Jothinagar AEE/Town/North/ 4 Cuddalore #172,Bharathiyar Street, Kondur, 04142- 225229 8 256 256 Cuddalore Cuddalore -2 AE/O&M,Annamalainagar, No.50, IV 5 Chidambaram AEE/Town/ Chidambaram North Cross, Mariappa Nagar 04144- 239322 7 256 256 ,Annamalai Nagar AE/O&M,AE/Rural/North/ Virudhachalam 110/11KV Vrd SS 6 Vridhachalam AEE/Rural/ Vridhachalam Campus,Budhamur,Vridhachalam- 04143-231971 4 256 256 606001 AE/O&M,AE/Urban/ Kandiyankuppam 110/11KV Vrd SS 7 Vridhachalam AEE/Town/ Vridhachalam Campus, Budhamur,Vridhachalam- 04143-238274 6 256 256 606001. 9445856076 AE/O&M,Rural Nellikuppam 10, Vazhapattu, 8 Nellikuppam AEE/Town/ Nellikuppam 04142-271699 4 256 256 Thirukandeeswaram AE/O&M,Melpattampakam #12A ,Market 9 Nellikuppam AEE/Town/ Nellikuppam st,Melpattampakkam,607104 04142-276017 5 256 256 Assistant Engineer/Town I/ Villupuram, 110/11KV/SS -

Biodiversity on Aquatic Microphytes Microphytes in Veeranam Tank
z Available online at http://www.journalcra.com INTERNATIONAL JOURNAL OF CURRENT RESEARCH International Journal of Current Research Vol. 5, Issue, 02, pp.336-353, February, 2013 ISSN: 0975-833X RESEARCH ARTICLE Biodiversity on Aquatic Microphytes in Veeranam Tank, Cuddalore District, Tamil Nadu Thangadurai, R., *Ravimycin, T. and Lenin, M. Ecology and Environmental Lab, Department of Botany Annamalai University, Annamalai Nagar-608 002 Department of Botany Government Arts College, Dharmapuri- 636805 ARTICLE INFO ABSTRACT Article History: In the present investigation, 140 species of phytoplankton belonging to 4 classes, viz., Cyanophyceae, Received 29th November, 2012 Chlorophyceae, Bacillariophyceae and Euglenophyceae were recorded. They belonged to 46 genera and 11 Received in revised form orders. Among the phytoplankton population, Bacillariophyceae constituted 40%, followed by Chlorophyceae 16th December, 2012 (30%), Cyanophyceae (20%) and Euglenophyceae (10%). The Bacillariophyceae was the dominant group of the Accepted 22th January, 2013 phytoplankton population in all the stations followed by Chlorophyceae, Cyanophyceae and Euglenophyceae. The th main reason for the high Bacillariophyceae population (40%) was rich nutrient and high temperature found in the Published online 14 February, 2013 tank. A total of 19 species belonging to 14 genera (Oscillatoria, Anabaena, Ankistrodesmus, Chlorella, Pediastrum, Scnedesmus, Tetraedron, Gomphonema, Navicula, Nitzschia, Synedra, Euglena, Phacus and Key words: Coelastrum) were recorded in all the stations. The maximum species was recorded in S-3 and S-4 due to the Biodiversity, pollution status in this station. Bacillariophyceae, Chlorophyceae, Cyanophyceae and Copy Right, IJCR, 2013, Academic Journals. All rights reserved. Euglenophyceae. INTRODUCTION composition respond to change of water quality in all perennial water bodies. The composition of algal diversity serves as an Biodiversity means the variability among living organisms from all important tool to analyse the water quality [5]. -

DR. NAME Father's /Husband Name
TAMILNADU STATE VETERINARY COUNCIL, CHENNAI-600035. DRAFT ELECTORAL ROLL-2013 SVPR Roll. Father's /Husband TNSVC SVPR SVPR PAGE.N No: DR. NAME Name ADDRESS Reg.No: YEAR Sl.NO: O: 44 /183-3, PUSHPAGAM EAST YMR 1 SAIRABANU S. P. SAMSUDEEN PATTI, DINDIGUL -624001. 2 2002 2 1 25 / 32A, KUNJAN VILAI, MANIKATTIPOTTAL (P.O.), 2 RAMESH S. R.SUYAMBU NAGERCOIL 629 501 3 2002 3 1 27, CHELLA PERUMAL ST., K.G.SUBRAMANIA SHOLINGHUR 631 102, VELLORE 3 VIJAYAKUMAR K. S. N DISTRICT 4 2002 4 1 # 220, METTU STREET, SAMPATH K.R.KARUNAKAR MANSION, NATHAM P.O., 4 SAMPATH K. AN CHENGALPATTU 603 001 5 2002 5 2 156D/163B, Subasri Nagar, Extn.I, 5 KAMALRAJ V. D. VENKATESAN Porur, Chennai - 600 0116 6 2002 6 2 ANAIPALAYAM (P.O.) ANDAGALUR GATE (VIA), RASIPURAM (TK), 6 LAVANYA K. A.KAILASAM NAMAKKAL DT., 637 401 7 2002 7 2 KEELA RADHA VEEDI, MUDUKULATHUR 623 704 , 7 KANNAN ALPADI A. T.T.ALPADI RAMANATHAPURAM DT., 8 2002 8 2 102, ARANI KOOT ROAD, PADMAVATHY A. W/o. A. KAMALA CHEYYAR - 604407 8 KANNAN THIRUVANNAMALAI DIST. 9 2002 9 3 122, MAIN ROAD. OLAGADAM 638 9 GANAPATHI RAJ M. R.MURUGESAN 314, ERODE DISTRICT 10 2002 10 3 OLD NO. 8,9 NEW NO. 5, RATHINAM R.GOVINDARAJA STREET, FIRST LANE, NEAR FIVE 10 DHANARAJ G. N CORNER, COIMBATORE-641001. 11 2002 11 3 15 / 1, MURUGA BHAVANAM, FIRST STREET, KAKKAN NAGAR, SURESH I. PALAYAMKOTTAI 11 S. IYYAPILLAI 627 353 12 2002 12 3 NO.17 & 19, FOURTH STREET, GOVINDA SWAMY NAGAR, KANDANCHAVADI, MADRAS 600 12 SARASWATHI M. -

Cuddalore Sl
CUDDALORE SL. NO. APPLICATION NO. NAME AND ADDRESS ARIVUKKARASAN. K S/O KUMAR NO.1/64, MAIN ROAD, 1 5780 ORATHUR POST, CHIDAMBARAM TALUK, CUDDALORE 608201 KARTHICK. K C-16, NSC BOSE ROAD, 2 5781 BLOCK - 11, NEYVELI , CUDDALORE SUGUNA. B 161C,ARUNTHATHIYAR STREET, VALLA PADUGAI, 3 5782 VALLATHURAI POST, CHIDAMBARAM, CUDDALORE 608401 KAVITHA . D D/O DHARMARASU 4 5783 80 CHIDAMBARAM MAIN ROAD, VADALUR, CUDDALORE 607303 KALAISELVAN. R KEELIRUPPU (EAST STREET), 5 5784 KADAMPULIYUR (VIA), PANRUTI TALUK, CUDDALORE 607103 RAMESH. K A-13, GREEN GRASS STREET, 6 5785 BLOCK-20, NEYVELI, CUDDALORE 607803 SINDHAN. S 315, DR.AMBEDKAR NAGAR, THERKKUVELLORE, 7 5786 OOMANGALAM POST, VRIDHACHALAM TALUK, CUDDALORE 607801 MAHENDRAN.R S/O RAMALINGAM AMMANKOVIL STREET, 8 5787 VETTAKUDI VILLAGE, T.V.PUTHUR (PO), VRIDHACHALAM (TK), CUDDALORE 606110 Page 1 SHANMUGAM. T NO.28, A.KUCHI PALAYAM, KEEL ARUNGUNAM (PO), 9 5788 PANRUTI (TK), NELLIKUPPAM, CUDDALORE 607105 THANGADURAI. B C.T.C. STREET, 10 5789 MELAKUPPAM POST, VRIDHACHALAM, CUDDALORE 607802 SIVABALAN.S NO.296/A2, ALADI ROAD, 11 5790 EAST KAMBAR ST, KAMARAJ NAGAR, VIRUDHACHALAM, CUDDALORE 606001 SENTHIL MURUGAN. A NO.518, MARIYAMMAN KOIL ST, 12 5791 KOTTLAMPAKKAM, PUDUPETTAI, PANRUT, CUDDALORE 607108 GNANADOSS. B NO.12, VALLUVAR STREET, 13 5792 UNNAMALAI CHETTY CHAVADI, KONDUR POST, CUDDALORE 607006 BABU. M 5/3, AMMAN KOVIL STREET, 14 5793 BHUDHAMUR, VRIDDHACHALAM TALUK, CUDDALORE 606001 SUKUMAR. R B-22, PARK CLUB STREET, 15 5794 BLOCK-25, NEYVELI-3, CUDDALORE 607803 VINAYAGA MOORTHY. S S/O G.SUBRAMANIYAN K.K.NAGAR, 16 5795 KILIMANGALAM POST, TITTAGUDI TK, CUDDALORE 606105 Page 2 RAJENDRAN. M S/O MAYAVAN. -

Full Length Research Article SEASONAL VARIATIONS OF
International Journal of Current Research in Life Sciences Vol. 05, Issue. 06, pp. 551-555, June 2016 www.ijcrls.com Full Length Research Article SEASONAL VARIATIONS OF PHYSICO-CHEMICAL CHARACTERISTICS IN VEERANAM LAKE CUDDALORE DISTRICT, TAMIL NADU, INDIA *Ronald Ross Department of Zoology, Annamalai University, Tamil Nadu, India, Annamalai Nagar -608002, India nd th Accepted 22 May 2016; Published Online 30 June 2016 ABSTRACT Seasonal variations of physico-chemical characteristics were carried out in Veeranam lake Kattumannarkovil in Cuddalore District, Tamil Nadu, India, for a period of twelve months (January 2017 to December 2017). Nine various physico-chemical parameters were analyzed by using standard methods (APHA, 1998). Water temperature varied from 28.55 to 32.52ºC, pH ranged from 7.23 to 8.28. Turbidity was varied between 26.48 to 40.38 cm and salinity was noted (0.07 to 0.16 ppt). Dissolved oxygen content varied from 4.71 to 5.71 mg/L, calcium (37.56 to 58.36 mg/L), phosphate (0.14 to 0.31), nitrate (0.21 to 0.29 mg/L) and ammonia (0.28 to 0.37 mg/L) also varied independently. Key words: Physico-chemical characteristics, Seasonal variations, Freshwater, Water quality. INTRODUCTION (Bhalerao, 2012; Pradeep et al., 2012; Wan Mohd Khalik et al., 2015). Water quality is the determining factor on the Water is the most abundant as well as a critical resource in success or failure of an aquaculture operation. The quality of nature and covers approximately 3⁄4th of the earth’s surface, water in any ecosystem provides significant information about although being so abundant many factors have contributed for the available resources for supporting life in that ecosystem. -
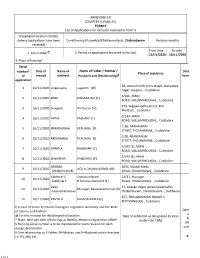
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applica Ons for Inclusion Received in Form 6 Designated Loca on Iden Ty (Where
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applicaons for inclusion received in Form 6 Designated locaon identy (where applicaons have been Constuency (Assembly/£Parliamentary): Chidambaram Revision identy received) From date To date 1. List number @ 2. Period of applicaons (covered in this list) 16/11/2020 16/11/2020 3. Place of hearing* Serial number $ Date of Name of Name of Father / Mother / Date of Place of residence of receipt claimant Husband and (Relaonship) # hearing* applicaon 28, second north cross street, mariyappa 1 16/11/2020 singaravelu suganthi (W) nagar, usuppur, , Cuddalore 2/106, MAIN 2 16/11/2020 ROJA NADARAJAN (F) ROAD, VALLAMPADUGAI, , Cuddalore 670, Sagajanandha Street, M K 3 16/11/2020 Sivagami Archunan (H) Thoam, , Cuddalore 2/144, MAIN 4 16/11/2020 ARTHI PAZHANI (F) ROAD, VALLAMPADUGAI, , Cuddalore C-28, ARANMANAI 5 16/11/2020 BARATHIRAJA PERUMAL (F) STREET, PICHAVARAM, , Cuddalore C,28, ARANMANAI 6 16/11/2020 KRISHNARAJ PERUMAL (F) STREET, PICHAVARAM, , Cuddalore 2/145 (3), MAIN 7 16/11/2020 APARNA PANDIYAN (F) ROAD, VALLAMPADUGAI, , Cuddalore 2/145 (3), MAIN 8 16/11/2020 ISWARYHA PANDIYAN (F) ROAD, VALLAMPADUGAI, , Cuddalore SRIRAM 1094, Vaikkal Meu 9 16/11/2020 AGILA GNANAVADIVEL (M) GNANAVADIVEL Street, Chidambaram, , Cuddalore Sakthivel S Somasundaram 23/31, Murugan 10 16/11/2020 Sakthivel S R Somasundaram R (F) Street, Chidambaram, , Cuddalore baby 31, velavan nagar, paramaswarnallur, 11 16/11/2020 Murugan Balasubramanian (O) balasubramanian Chidambaram, Chidambaram, , Cuddalore 357, THILLAIAMMAN