Ight © Tarek Kakhia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Controlled/Living Radical Polymerization in Aqueous Media: Homogeneous and Heterogeneous Systems
Prog. Polym. Sci. 26 =2001) 2083±2134 www.elsevier.com/locate/ppolysci Controlled/living radical polymerization in aqueous media: homogeneous and heterogeneous systems Jian Qiua, Bernadette Charleuxb,*, KrzysztofMatyjaszewski a aDepartment of Chemistry, Center for Macromolecular Engineering, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, PA 15213, USA bLaboratoire de Chimie MacromoleÂculaire, Unite Mixte associeÂe au CNRS, UMR 7610, Universite Pierre et Marie Curie, Tour 44, 1er eÂtage, 4, Place Jussieu, 75252 Paris cedex 05, France Received 27 July 2001; accepted 30 August 2001 Abstract Controlled/living radical polymerizations carried out in the presence ofwater have been examined. These aqueous systems include both the homogeneous solutions and the various heterogeneous media, namely disper- sion, suspension, emulsion and miniemulsion. Among them, the most common methods allowing control ofthe radical polymerization, such as nitroxide-mediated polymerization, atom transfer radical polymerization and reversible transfer, are presented in detail. q 2001 Elsevier Science Ltd. All rights reserved. Keywords: Aqueous solution; Suspension; Emulsion; Miniemulsion; Nitroxide; Atom transfer radical polymerization; Reversible transfer; Reversible addition-fragmentation transfer Contents 1. Introduction ..................................................................2084 2. General aspects ofconventional radical polymerization in aqueous media .....................2089 2.1. Homogeneous polymerization .................................................2089 -

Defoamer Formulation Entschäumerzusammensetzung Composition Antimousse
(19) TZZ ¥ZZ_T (11) EP 2 430 095 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08L 33/06 (2006.01) B01D 19/04 (2006.01) 07.03.2018 Bulletin 2018/10 (86) International application number: (21) Application number: 10720061.0 PCT/US2010/033827 (22) Date of filing: 06.05.2010 (87) International publication number: WO 2010/132262 (18.11.2010 Gazette 2010/46) (54) DEFOAMER FORMULATION ENTSCHÄUMERZUSAMMENSETZUNG COMPOSITION ANTIMOUSSE (84) Designated Contracting States: • WILSON, Robert AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Marietta, GA 30060 (US) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • ROSENCRANCE, Scott PL PT RO SE SI SK SM TR Douglasville, GA 30135 (US) • PREVIS, David (30) Priority: 15.05.2009 US 466637 King William, VA 23086 (US) (43) Date of publication of application: (74) Representative: Potter Clarkson LLP 21.03.2012 Bulletin 2012/12 The Belgrave Centre Talbot Street (73) Proprietor: Kemira Chemicals Inc. Nottingham NG1 5GG (GB) Atlanta GA 30339 (US) (56) References cited: (72) Inventors: GB-A- 2 094 330 US-A- 5 152 925 • MARTIN, James Bonaire, GA 31005 (US) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. Notice of opposition shall not be deemed to have been filed until the opposition fee has been paid. -

Thieves® Whitening Toothpaste
THIEVES® WHITENING TOOTHPASTE PRODUCT SUMMARY Start your morning on the bright side with the natural power of Thieves® Whitening Toothpaste. Pure and safe ingredients combine to whiten teeth, fight plaque, support healthy gums, and remove stains without damaging enamel. Great for the whole family, Young Living’s exclusive formula is both fluoride free and free of other harsh ingredients, offering you a superior clean you can be confident in. Grin from ear to ear knowing you made a fresh choice with Thieves Whitening Toothpaste! KEY INGREDIENTS Calcium hydroxyapatite, Silica, Perlite, Xylitol, Calcium bicarbonate, Thieves essential oil blend, Spearmint essential oil, Peppermint essential oil, Orange essential oil EXPERIENCE BENEFITS & FEATURES Smile bright with Thieves Whitening Toothpaste. • Whitens and brightens teeth Thieves fans will fall in love with this fresh take on Thieves-infused toothpaste. Fresh Peppermint, • Gently removes surface stains and cleans teeth cool Spearmint, and zesty Orange essential oils • Free from harmful peroxides combine with our spicy Thieves blend to leave your breath EO-fresh all day. Pure and safe whitening • Helps reduce and prevent tartar buildup agents leave you with long-lasting freshness and • Formulated without fluoride, SLS, parabens, a sparkling smile! phthalates, mineral oil, synthetic perfumes or dyes, toxic ingredients, or artificial colors, flavors, or preservatives PRODUCT BACKGROUND • Fights plaque and supports healthy gums Thieves Whitening Toothpaste is one more way Young Living is bringing the power of nature to your • Provides a long-lasting, smooth, fresh feel daily routine: a toothpaste gentle enough for every • Fluoride free and cruelty free day, formulated with essential oils and other natural • Formulated without GMOs ingredients. -

The Activated Sludge Process Part 1 Revised July 2014
Wastewater Treatment Plant Operator Certification Training Module 15: The Activated Sludge Process Part 1 Revised July 2014 This course includes content developed by the Pennsylvania Department of Environmental Protection (Pa. DEP) in cooperation with the following contractors, subcontractors, or grantees: The Pennsylvania State Association of Township Supervisors (PSATS) Gannett Fleming, Inc. Dering Consulting Group Penn State Harrisburg Environmental Training Center MODULE 15: THE ACTIVATED SLUDGE PROCESS - PART 1 Topical Outline Unit 1 – General Description of the Activated Sludge Process I. Definitions A. Activated Sludge B. Activated Sludge Process II. The Activated Sludge Process Description A. Organisms B. Secondary Clarification C. Activated Sludge Process Control III. Activated Sludge Plants A. Types of Plants B. Factors that Upset Plant Operation IV. Unit Review V. References Unit 2 – Aeration I. Purpose of Aeration II. Aeration Methods A. Mechanical B. Diffused III. Aeration Systems A. Mechanical Aeration Systems B. Diffused Aeration Systems Bureau of Safe Drinking Water, Department of Environmental Protection Wastewater Treatment Plant Operator Training i MODULE 15: THE ACTIVATED SLUDGE PROCESS - PART 1 IV. Safety Procedures A. Aeration Tanks and Clarifiers B. Surface Aerators C. Air Filters D. Blowers E. Air Distribution System F. Air Headers and Diffusers V. Review VI. References Unit 3 – New Plant Start-Up Procedures I. Purpose of Plant and Equipment Review A. Document Familiarization B. Equipment Familiarization II. Equipment and Structures Check A. Flow Control Gates and Valves B. Piping and Channels C. Weirs D. Froth Control System E. Air System F. Secondary Clarifier III. Process Start-Up A. Process Units B. Process Control IV. Unit Review V. -

Labels of Pesticides Used with This Product
Brandt® Organics Defoamer Antifoaming / Defoaming Agent KEEP OUT OF REACH OF CHILDREN Principal Functioning Agents: Silica filled polydimethylsiloxane .................10% WARNING Constituents ineffective as spray adjuvant ....... 90% Total........................................... 100% PRECAUTIONARY STATEMENTS CA Reg. No. 48813-50022-AA HAZARDS TO HUMANS AND DOMESTIC ANIMALS DIRECTIONS FOR USE WARNING: Causes serious eye irritation. Causes skin irritation. Wear protective eyewear, long sleeved shirt, long pants, shoes BRANDT ORGANICS DEFOAMER is a high quality silicone based plus socks and chemical resistant gloves made of any waterproof anti-foaming agent designed to prevent foam formation or defeat material when mixing or handling concentrate. Wash thoroughly existing foams in pesticide spray solutions. Excessive foaming with soap and water after handling and before eating, drinking, can promote inefficiency in spray activities. It may be used on chewing gum, using tobacco, or using the toilet. Remove and wash agricultural, forestry, turf and ornamental, industrial, structural contaminated clothing before reuse. and non-cropland sites. Not for aquatic use. Read and follow the precautions, restrictions and recommendations ENVIRONMENTAL HAZARDS on the labels of pesticides used with this product. Use according to Do not contaminate water when cleaning equipment or disposing the most restrictive label directions for each product in any tank mix. of equipment washwaters. COMPATIBILITY: BRANDT ORGANICS DEFOAMER is compatible FIRST AID with most pesticides. However, if the desired combination has Have the product container or label with you when calling a poison not been previously used, a compatibility test is recommended. control center or doctor or going for treatment. MIXING: Fill spray tank 1/2 full of water and begin agitation. -

Glossary: Wastewater Treatment and Collection System Terms
Glossary September 2011 Wastewater Treatment Collection System &Terms Minnesota Pollution Control Agency 520 Lafayette Road North St. Paul, Minnesota 55155-4194 wq-wwtp8-20 Contents Wastewater Treatment and Collection System Terms A ........... 3-6 G ...... 24-25 M ..... 29-31 S ........ 42-51 B ........... 7-9 H ....... 26-27 N ....... 32-33 T ........ 52-54 C ....... 10-15 I ......... 27-28 O ...... 33-35 U ............ 54 D ....... 15-19 J ............. 28 P ....... 35-40 V ....... 55-56 E ........ 19-21 K ............ 28 Q ............ 40 W ...... 56-57 F ........ 21-24 L ............. 29 R ....... 40-42 Z ............. 57 Acknowledgement Special thanks to Ken Kerri at California State University, Sacramento, for use of the glossary that appears in his self-study training courses, “Operation and Maintenance of Wastewater Collection Systems” and “Operation of Wastewater Treatment Plants.” A special acknowledgement to the Water Environment Federation for the use of their wastewater glossary for some of the definitions in this manual. Contributors Kay Curtin Steve Duerre Gene Erickson Editing and Graphic Design Nancy Ellefson This document was printed on 30 percent post-consumer recycled paper manufactured without the use of elemental chlorine. 2 Minnesota Pollution Control Agency | All rights reserved. Wastewater Treatment and Collection System Terms A absorption The taking up of one substance into the body of another by chemical or molecular action after the adsorbtion process. accuracy In physical measurements, it is the degree of agreement between the quantity measured and the actual quantity. It should not be confused with ‘precision,’ which denotes the reproducibility of the measurement. acid A substance that yields hydrogen ions when dissolved in water resulting in a pH of less than 7; it can react with bases and certain metals to form salts. -

Effects of Solvent Treatment and High-Pressure Homogenization Process on Dispersion Properties of Palygorskite
Powder Technology 235 (2013) 652–660 Contents lists available at SciVerse ScienceDirect Powder Technology journal homepage: www.elsevier.com/locate/powtec Effects of solvent treatment and high-pressure homogenization process on dispersion properties of palygorskite Jixiang Xu a,b, Wenbo Wang a,c, Aiqin Wang a,c,⁎ a Center of Eco-material and Green Chemistry, Lanzhou Institute of Chemical Physics, Chinese Academy of Science, Lanzhou 730000, China b Graduate University of the Chinese Academy of Science, Beijing 100049, China c R&D Center of Xuyi Palygorskite Applied Technology, Lanzhou Institute of Chemical Physics, Chinese Academy of Science, Xuyi 211700, China article info abstract Article history: Dispersion of natural palygorskite in distilled water, methanol, ethanol, isopropanol, dimethyl formamide, Received 12 September 2012 and dimethyl sulfoxide was carried out using high-pressure homogenization. The effects of solvent parame- Received in revised form 20 September 2012 ters on the microstructure, morphology and colloidal properties of palygorskite were investigated in detail. Accepted 19 November 2012 Elemental analysis, infrared spectroscopy (IR) and thermogravimetric analysis (TGA) confirmed that some Available online 27 November 2012 solvent molecules were encapsulated within the tunnels of palygorskite. The efficiency of the homogeniza- tion process to disperse palygorskite aggregates was closely correlated to the solvent parameters, particularly Keywords: Palygorskite solvent vapor pressure and viscosity. A well disaggregated palygorskite was obtained after dispersing in di- Dispersion methyl sulfoxide. It was also confirmed that colloidal stability and suspension viscosity were affected by Solvents the solvent nature. A more stable suspension with higher viscosity of 2364 mPa s was obtained by dispersing Homogenization palygorskite in isopropanol. -

Atom Transfer Radical Polymerization in Aqueous Dispersed Media
Cent. Eur. J. Chem. • 7(4) • 2009 • 657–674 DOI: 10.2478/s11532-009-0092-1 Central European Journal of Chemistry Atom transfer radical polymerization in aqueous dispersed media Invited review Ke Min, Krzysztof Matyjaszewski* Center for Macromolecular Engineering, Department of Chemistry, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh PA 15213 Received 1 June 2009; Accepted 3 August 2009 Abstract: During the last decade, atom transfer radical polymerization (ATRP) received significant attention due to its exceptional capability of synthesizing polymers with pre-determined molecular weight, well-defined molecular architectures and various functionalities. It is economically and environmentally attractive to adopt ATRP to aqueous dispersed media, although the process is challenging. This review summarizes recent developments of conducting ATRP in aqueous dispersed media. The issues related to retaining “controlled/living” character as well as colloidal stability during the polymerization have to be considered. Better understanding the ATRP mechanism and development of new initiation techniques, such as activators generated by electron transfer (AGET) significantly facilitated ATRP in aqueous systems. This review covers the most important progress of ATRP in dispersed media from 1998 to 2009, including miniemulsion, microemulsion, emulsion, suspension and dispersed polymerization. Keywords: ATRP • Controlled radical polymerization • Dispersed media • Emulsion © Versita Warsaw and Springer-Verlag Berlin Heidelberg. 1. Introduction and scope of the media, preferably with water as the continuous phase. review Aqueous dispersed media are commonly recognized as a diverse and benign means for conducting industrial It has been a long-standing goal in polymer chemistry scale polymerization. The use of water as the dispersion to develop a process that combines the robustness medium is environmentally friendly, as compared to of radical polymerization with precise control over using volatile organic solvents. -

Predicting Defoamer Performance in Coating Formulations
Predicting Defoamer Performance in Coating Formulations C. James Reader and K.T. Griffin Lai Air Products and Chemicals, Inc., 7201 Hamilton Boulevard, Allentown, PA 18195-1501 C. James Reader, [email protected], 610-481-7380 K.T. Griffin Lai, [email protected], 610-481-7069 Introduction What is the usual procedure for finding a defoamer for a new waterborne coating formulation? For many companies, the procedure appears to be as follows: try the defoamers that you currently use and know; if these don’t work then try samples in the lab, ask a colleague or friend or, maybe, ask a supplier. This approach makes good sense when working with formulations that are similar, as defoamers will usually give consistent performance in similar formulations. However, when these tried and trusted defoamers don’t work, the Chemist has the frustrating task of trying to find a suitable product from a large selection of mysterious bottles on his or her lab bench. There is a truism in England that goes “if all else fails, read the instructions”; however, defoamers don’t usually come with instructions. Wouldn’t it be nice if they did? The difficulties in finding a suitable defoamer exist because the performance of each and every defoamer is affected by the formulation it is used in; change the formulation and the defoamer performance may often change as well. Defoamer selection is also one of the last steps in readying a formulation for final use, so the rest of the formulation is already mostly decided and the defoamer has to work with this. -

MSDS for Anti-Foaming Agent
MATERIAL SAFETY DATA SHEET for Anti-Foaming Agent Revision: 1.0 1/19/2005 Page 1 of 8 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Anti-Foaming Agent Chemical Name: Polydimethylsiloxane emulsion Supplier: The Real Milk Paint Co. 11 W. Pumping Station Rd. Quakertown, PA 18951 Contact Numbers: CHEM-TELL (24 Hours) 800-255-0573 Customer Service 800-339-9748 2. COMPOSITION/INFORMATION ON INGREDIENTS COMPOENT CAS3 CONCENTRATION Water 7732-18-5 >50.0% Proprietary additives Trade Secret <30.0% Silica filled polydimethylsiloxane Trade Secret <15.0% Note(s): See Section 15 for chemicals appearing on Federal or State Right-TO-Know lists. 3. HAZARDOUS IDENTIFICATION EMERGENCY OVERVIEW NORMAL PRECAUTIONS COMMON TO SAFE MANUFACTURING PRACTICE SHOULD BE FOLLOWED IN HANDLING AND STORAGE. 4. FIRST AID MEASURES Swallowing No emergency care anticipated Skin Wash skin with soap and water MATERIAL SAFETY DATA SHEET for Anti-Foaming Agent Revision: 1.0 1/19/2005 Page 2 of 8 Inhalation No emergency care anticipated. Eye contact Flush eyes thoroughly with water for several minutes Notes to physicians Toxicology studies have shown this material to be of very low acute toxicity. There is no specific antidote. Treatment of overexposure should be directed at the control of symptoms and the clinical condition of the patient. 5. FIRE FIGHTING MEASURES Flammable limits Lower limits Not available Upper limits Not available Special fire fighting procedures None. Special protective equipment for firefighters Use self-contained breathing apparatus when fighting fires in enclosed areas. Extinguishing media Suitable: Non-flammable (aqueous) After water evaporates, the remaining material will burn. Large fires: -Alcohol-type foam or universal type foam Small fires: -CO2 -dry chemical Unsuitable: None. -

Injection Molding Foam Guidelines
SAFOAM USER’S GUIDE This ‘SAFOAM User’s guide’ was prepared as a service to the customers using Reedy International Corporation’s SAFOAM family of endothermic foaming agents. Reproduction or distribution of this brochure, in whole or in part, is prohibited without the permission of Reedy International Corporation. TABLE OF CONTENTS 1. Introduction ........................................................................................................1 SAFOAM - Its Use in Thermoplastics ........................................................2 2. Structural Plastics and Polymer Selection .........................................................3 Properties of Amorphous Polymers .............................................................3 Properties of Crystalline Polymers ..............................................................4 Properties of Thermoplastic Elastomers ......................................................4 3. Foaming Agent Selection ...................................................................................6 Physical Foaming Agents ............................................................................7 Chemical Foaming Agents ...........................................................................7 Exothermic Chemical Foaming Agents Azodicarbonamide .................................................................................8 Endothermic Chemical Foaming Agents Sodium Borohydride ..............................................................................9 Traditional Acid/Carbonate Systems -
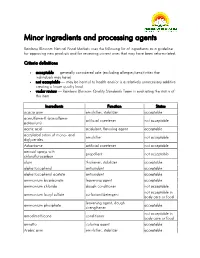
Minor Ingredients and Processing Agents
Minor ingredients and processing agents Rainbow Blossom Natural Food Markets uses the following list of ingredients as a guideline for approving new products and for reviewing current ones that may have been reformulated. Criteria definitions acceptable — generally considered safe (excluding allergies/sensitivities that individuals may have) not acceptable — may be harmul to health and/or is a relatively unnecessary additive creating a lower quality food under review — Rainbow Blossom Quality Standards Team is evaluating the status of this item Ingredients Function Status acacia gum emulsifier, stabilizer acceptable acesulfame-K (acesulfame- artificial sweetener not acceptable potassium) acetic acid acidulant, flavoring agent acceptable acetylated esters of mono- and emulsifier not acceptable diglycerides Advantame artificial sweetener not acceptable aerosol sprays with propellant not acceptable chloroflurocarbon algin thickener, stabilizer acceptable alpha tocopherol antioxidant acceptable alpha tocopherol acetate antioxidant acceptable ammonium bicarbonate leavening agent acceptable ammonium chloride dough conditioner not acceptable not acceptable in ammonium lauryl sulfate surfactant/detergent body care or food leavening agent, dough ammonium phosphate acceptable strengthener not acceptable in amodimethicone conditioner body care or food annatto coloring agent acceptable Arabic gum emulsifier, stabilizer acceptable Ingredients Function Status artificial colors (red #3, red # 40, yellow #5, yellow #6, blue #1, blue coloring agent