06 Martens.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beidaihe^ China: East Asian Hotspot Paul I
Beidaihe^ China: East Asian hotspot Paul I. Holt, Graham P. Catley and David Tipling China has come a long way since 1958 when 'Sparrows [probably meaning any passerine], rats, bugs and flies' were proscribed as pests and a war declared on them. The extermination of a reputed 800,000 birds over three days in Beijing alone was apparently then followed by a plague of insects (Boswall 1986). After years of isolation and intellectual stagnation during the Cultural Revolution, China opened its doors to organised foreign tour groups in the late 1970s and to individual travellers from 1979 onwards. Whilst these initial 'pion eering' travellers included only a handful of birdwatchers, news of the country's ornithological riches soon spread and others were quick to follow. With a national avifauna in excess of 1,200 species, the People's Republic offers vast scope for study. Many of the species are endemic or nearly so, a majority are poorly known and a few possess an almost mythical draw for European birders. Sadly, all too many of the endemic forms are either rare or endangered. Initially, most of the recent visits by birders were via Hong Kong, and concentrated on China's mountainous southern and western regions. Inevitably, however, attention has shifted towards the coastal migration sites. Migration at one such, Beidaihe in Hebei Province, in Northeast China, had been studied and documented by a Danish scientist during the Second World War (Hemmingsen 1951; Hemmingsen & Guildal 1968). It became the focus of renewed interest after a 1985 Cambridge University expedition (Williams et al. -

Download PDF # Sylviidae: Chrysomma, Fulvetta, Paradoxornis
NINOGGSXCNNZ \\ Doc > Sylviidae: Chrysomma, Fulvetta, Paradoxornis, Parisoma, Sylvia, Parrotbill, Old World warbler, Jerdon's Babbler,... Sylviidae: Chrysomma, Fulvetta, Paradoxornis, Parisoma, Sylvia, Parrotbill, Old World warbler, Jerdon's Babbler, Wrentit, Blackcap Filesize: 6.59 MB Reviews Very beneficial to all type of folks. I could comprehended every thing using this created e pdf. I found out this book from my i and dad suggested this book to find out. (Ms. Madaline Nienow) DISCLAIMER | DMCA GPPUH64MHWS0 < eBook \\ Sylviidae: Chrysomma, Fulvetta, Paradoxornis, Parisoma, Sylvia, Parrotbill, Old World warbler, Jerdon's Babbler,... SYLVIIDAE: CHRYSOMMA, FULVETTA, PARADOXORNIS, PARISOMA, SYLVIA, PARROTBILL, OLD WORLD WARBLER, JERDON'S BABBLER, WRENTIT, BLACKCAP To download Sylviidae: Chrysomma, Fulvetta, Paradoxornis, Parisoma, Sylvia, Parrotbill, Old World warbler, Jerdon's Babbler, Wrentit, Blackcap PDF, please access the hyperlink listed below and save the document or have access to additional information that are have conjunction with SYLVIIDAE: CHRYSOMMA, FULVETTA, PARADOXORNIS, PARISOMA, SYLVIA, PARROTBILL, OLD WORLD WARBLER, JERDON'S BABBLER, WRENTIT, BLACKCAP ebook. Books LLC, Wiki Series, 2016. Paperback. Book Condition: New. PRINT ON DEMAND Book; New; Publication Year 2016; Not Signed; Fast Shipping from the UK. No. book. Read Sylviidae: Chrysomma, Fulvetta, Paradoxornis, Parisoma, Sylvia, Parrotbill, Old World warbler, Jerdon's Babbler, Wrentit, Blackcap Online Download PDF Sylviidae: Chrysomma, Fulvetta, Paradoxornis, Parisoma, -

Thick-Billed Warbler (Iduna Aedon) at Gambell, Alaska: First Record for North America Gary H
NOTES THICK-BILLED WARBLER (IDUNA AEDON) AT GAMBELL, ALASKA: FIRST RECORD FOR NORTH AMERICA GARY H. ROSENBERG, 8101 North Wheatfield Dr., Tucson, Arizona 85741; [email protected] PAUL E. LEHMAN, 11192 Portobelo Dr., San Diego, California 92124; [email protected] AARON J. LANG, 40208 Alpenglow Circle, Homer, Alaska 99603; [email protected] VICTOR AND RUBEN STOLL, 899 Miller Rd., Centerville, Tennessee 37033; [email protected] In the evening on 8 September 2017, in the “far boneyard” at Gambell, St. Law- rence Island, Alaska (63.78° N, 171.74° W), Victor and Ruben Stoll flushed a pas- serine they could not immediately identify. The “boneyards” are large pits excavated by the resident Yupik Natives seeking buried ivory and artifacts, a result of several thousand years of sea-mammal hunting from this island’s Northwest Cape. Working these pits turns the soil, which has resulted in the growth of relatively lush vegetation consisting of two species of Artemisia, known locally as “wormwood.” The combina- tion of lush vegetation (reaching 0.5–1 m in height) and deep depressions that offer protection from the wind is attractive to migrant and vagrant landbirds in the otherwise flat, gravelly landscape. Soon thereafter, we, along with Greg Scyphers, Monte Taylor, and other birders then at Gambell, converged at the far boneyard in search of the bird. It was soon relocated and seen on the ground briefly by Lang, who suggested it was a Thick-billed Warbler (Iduna aedon), a bird he was familiar with from southeastern Asia and a species not previously recorded in Alaska or North America. -

Qinghai, China June 2010
Qinghai, China June 2010 June - July 2010 Bengt-Eric Sjölinder Qinghai 16.6-4.7 2010 Short Itinerary 16.6 Flight Beijing-Xining. 17.6 Dong Xya. Then via Datong to Xihay. 18.6 East Koko Nor, West Koko Nor to Heimahe. Night in Chaka. 19.6 Valley in the Dolan Mountains. Back to Chaka. 20.6 Desert E Chaka. The Gulag Grove and the Chaka Grove. Chaka Wadi, W Chaka. 21.6 Rubber Mountains. Then South Koko Nor Range to Gonghe. Valley close to Gonghe. 22.6 Continuing south. Stop at ”the Gully”. On to Er la Pass. Night in Wenquan. 23.6 Er la Pass. Wenquan in the evening. 24.6 Wenquan-Madoo-Dayematan-Bayankala Pass-Yushu. 25.6 Yushu-”the Gorge”. Road towards Nanquen Forest Reserve. Night in Nanquen. 26.6 South of Nanquen. ”the Pass” and ”the Spruce Forest”. Night in Nanquen. 27.6 Kanda Shan. 28.6 ”the Spruce Forest” south of Nanquen. 29.6 Nanquen-”the Gorge”-Yushu-the lake Longbaotan-Qumarleb. 30.6 Qumarleb-Qumahe-the main road between Golmud and Lhasa. Night in Golmud. 1.7 Golmud-Chaka with short stops. 2.7 Chaka-Heimahe-road north of Koko Nor-Xining. 3.7 Xining Beishan. Flight to Beijing in the evening. 4.7 Beijing-Copenhagen. Participants Jesper Hornskov (Tour guide; [email protected]) Ola Elleström Erik Hirschfeld Nils Kjellén (Bird and Mammal checklist) Mats Rellmar Elsy-Britt Schildt Bengt-Eric Sjölinder (Trip report including pictures unless noted as taken by Erik or Mats; [email protected]) Cover page photo Roborowski’s Rosefinch and Tibetan Sandgrouse (Erik) 2 Detailed Itinerary 16.6 We were five participants that boarded the direct SAS flight from Copenhagen to Beijing in the evening of the 15th and landed the following day in the Chinese capital at noon to catch our connecting 3pm flight to Xining where we landed at 6.30pm. -

Based on Nuclear and Mitochondrial Sequence Data
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 29 (2003) 126–138 www.elsevier.com/locate/ympev Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data Per G.P. Ericsona,* and Ulf S. Johanssona,b a Department of Vertebrate Zoology and Molecular Systematics Laboratory, Swedish Museum of Natural History, Frescativagen 44, P.O. Box 50007, SE-10405 Stockholm, Sweden b Department of Zoology, University of Stockholm, SE-106 91 Stockholm, Sweden Received 18 September 2002; revised 23 January 2003 Abstract Passerida is a monophyletic group of oscine passerines that includes almost 3500 species (about 36%) of all bird species in the world. The current understanding of higher-level relationships within Passerida is based on DNA–DNA hybridizations [C.G. Sibley, J.E. Ahlquist, Phylogeny and Classification of Birds, 1990, Yale University Press, New Haven, CT]. Our results are based on analyses of 3130 aligned nucleotide sequence data obtained from 48 ingroup and 13 outgroup genera. Three nuclear genes were sequenced: c-myc (498–510 bp), RAG-1 (930 bp), and myoglobin (693–722 bp), as well one mitochondrial gene; cytochrome b (879 bp). The data were analysed by parsimony, maximum-likelihood, and Bayesian inference. The African rockfowl and rock- jumper are found to constitute the deepest branch within Passerida, but relationships among the other taxa are poorly resolved— only four major clades receive statistical support. One clade corresponds to Passeroidea of [C.G. Sibley, B.L. Monroe, Distribution and Taxonomy of Birds of the World, 1990, Yale University Press, New Haven, CT] and includes, e.g., flowerpeckers, sunbirds, accentors, weavers, estrilds, wagtails, finches, and sparrows. -

Composition of Bird Species in Huu Lien Nature Reserve, Lang Son Province
VNU Journal of Science, Earth Sciences 27 (2011) 13-24 Composition of bird species in Huu Lien Nature Reserve, Lang Son province Nguyen Lan Hung Son*, Nguyen Thi Hoa, Le Trung Dung Hanoi National University of Education, 136 Xuan Thuy, Hanoi, Vietnam Received 3 December 2010; received in revised form 17 December 2010 Abstract . The diversity of bird species is of special importance as it can create responsive and adaptive behaviours among the whole animal population in wild environment. For this reason, the frequent making of inventory lists of bird species helps assess and evaluate the current status of forest resources in natural conservation areas which are inherently under human pressures in our country. During the two years (2009 - 2010) of the study conducted in Huu Lien Nature Reserve in Lang Son province, records have been made of 168 bird species belonging to 117 genus, 54 families, 17 orders. Of these, 9 bird species are rare and of high value of genetic preservation. Discussions have been held on the data for classification and arrangement of bird lists. This regional avifauna is characterized as typical of the lime stone mountain ecology in the Northeast of Vietnam along the border with China. The illegal activities of timbering Buretiodendron hsienmu take place at high frequency are making it a threat to the conservation of the diversity of bird species in this area. Keywords: avifauna, lime stone mountain, rare species, timbering. 1. Introduction ∗∗∗ hectares and an buffer zone of another 10.000 hectares. On May 31 st 2006, the Chairman of Huu Lien Nature Reserve was recognized as Lang Son People’s Committee issued the in the Decision numbered 194/CT dated August decision numbered 705/Q Đ-UBND on th 9 1986 by the Council of Ministers. -

14Th of November 2019
Date: 14th of november 2019 Tour: Half day excursion Oostvaardersplassen Guide: Pim Around 8.30 I met Quentin from Vancouver (Canada) at the small trainstation of Almere Centrum. From here we drove to the nature reserve Oostvaardersplassen. Our first stop was at a viewing point. From a distance we saw a large group of Red deer, on farmland we saw a few smaller Roe deer. We saw our first White tailed eagle of the day, his nickname is “flying door”, because his wingspan is about 2.30 meters! At the moment there are about 10-12 of this large birds in the area and we saw this morning half of this population. In The Netherlands there are about 10 breeding pairs, it al started in the Oostvaardersplassen about 13 years ago. We saw many Common buzzard, Common kestrel and a male Eurasian sparrowhaw was showing well closeby. We saw a large group of Barnacle geese and Greylag geese and a mixed group of hundreds of Northern lapwings and European golden plovers. Also a adult Whooper swan was present. Eurasian sparrowhawk – Oostvaardersplassen – Lelystad. “The whooper swan is a large white swan, bigger than a Bewick's swan. It has a long thin neck, which it usually holds erect, and black legs. Its black bill has a large triangular patch of yellow on it”. Source: Rspb.org. The Whooper swans we saw in the reserve today are mainly from Russia and Finland, a pair stays together for the rest of their live On our second stop, also a viewing point, we saw a juvenile White tailed eagle and two adults in a tree. -

AIRONE CENERINO Ardea Cinerea the Grey Heron (Ardea Cinerea
AIRONE CENERINO Ardea cinerea The Grey Heron (Ardea cinerea ) is a wading bird of the heron family Ardeidae . The Grey Heron is a large bird, standing 1 m tall, and it has a 1.5 m wingspan. It is the largest European heron. Its plumage is largely grey above, and off-white below. It has a powerful yellow bill, which is brighter in breeding adults. It has a slow flight, with its long neck retracted (S-shaped). This is characteristic of herons and bitterns, and distinguishes them from storks, cranes and spoonbills, which extend their necks This species breeds in colonies in trees close to lakes or other wetlands, although it will also nest in reed beds. It builds a bulky stick nest. It feeds in shallow water, spearing fish or frogs with its long, sharp bill. Herons will also take small mammals and birds. It will often wait motionless for prey, or slowly stalk its victim. The call is a loud croaking "fraaank". This species is very similar to the American Great Blue Heron. The Australian White-faced Heron is often incorrectly called Grey Heron ALLODOLA Alauda arvensis The Skylark (Alauda arvensis ) is a small passerine bird. It breeds across most of Europe and Asia and in the mountains of north Africa. It is mainly resident in the west of its range, but eastern populations of are more migratory, moving further south in winter. Even in the milder west of its range, many birds move to lowlands and the coast in winter. Asian birds appear as vagrants in Alaska; this bird has also been introduced in Hawaii and western North America. -

This Dissertation Has Been 61-3328 Microfilmed Exactly As Received
THE TAXONOMIC SIGNIFICANCE OF THE TONGUE MUSCULATURE OF PASSERINE BIRDS Item Type text; Dissertation-Reproduction (electronic) Authors George, William Gordon, 1925- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 05/10/2021 15:17:28 Link to Item http://hdl.handle.net/10150/284412 This dissertation has been 61-3328 microfilmed exactly as received GEORGE, William Gordon, 1925- THE TAXONOMIC SIGNIFICANCE OF THE TONGUE MUSCULATURE OF PASSERINE BIRDS. University of Arizona, Ph.D., 1961 Zoology University Microfilms, Inc., Ann Arbor, Michigan THE TAXONOMIC SIGNIFICANCE OF THE TONGUE MUSCULATURE OF PASSERINE BIRDS by William G. George A Dissertation Submitted to the Faculty of the DEPARTMENT OF ZOOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 1961 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE I hereby recommend that this dissertation prepared under my direction by Wllllaa G. George entitled The Taxonoalc Significance of the Tongue Musculature of Passerine Birds be accepted as fulfilling the dissertation requirement of the degree of Doctor of Philosophy Dissertation Director / Date After inspection of the dissertation, the following members of the Final Examination Committee concur in its approval and recommend its acceptance:* *This approval and acceptance Is contingent on the candidate's adequate performance and defense of this dissertation at the final oral examination. -
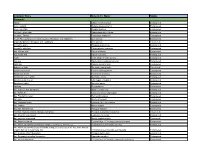
List of Endangered Species
Common Name Scienticfic Name Status Mammals Addax Addax nasomaculatus Endangered Anoa, lowland Bubalus depressicornis Endangered Anoa, mountain Bubalus quarlesi Endangered Antelope, giant sable Hippotragus niger variani Endangered Antelope, Tibetan Panthalops hodgsonii Endangered Argali [All populations except Kyrgyzstan, Mongolia, and Tajikistan] Ovis ammon Endangered Argali [Kyrgyzstan, Mongolia, and Tajikistan] Ovis ammon Threatened Armadillo, giant Priodontes maximus Endangered Armadillo, pink fairy Chlamyphorus truncatus Endangered Ass, African wild Equus africanus Endangered Ass, Asian wild Equus hemionus Endangered Avahi Avahi laniger(=entire genus) Endangered Aye-aye Daubentonia madagascariensis Endangered Babirusa Babyrousa babyrussa Endangered Baboon, gelada Theropithecus gelada Threatened Bandicoot, barred Perameles bougainville Endangered Bandicoot, desert Perameles eremiana Endangered Bandicoot, lesser rabbit Macrotis leucura Endangered Bandicoot, pig-footed Chaeropus ecaudatus Endangered Bandicoot, rabbit Macrotis lagotis Endangered Banteng Bos javanicus Endangered Bat, Bulmer's fruit (flying fox) Aproteles bulmerae Endangered Bat, bumblebee Craseonycteris thonglongyai Endangered Bat, Florida bonneted Eumops floridanus Endangered Bat, gray Myotis grisescens Endangered Bat, Hawaiian hoary Lasiurus cinereus semotus Endangered Bat, Indiana Myotis sodalis Endangered Bat, little Mariana fruit Pteropus tokudae Endangered Fruit Bat, Mariana (=fanihi, Mariana flying fox) Pteropus mariannus mariannus Threatened Bat, Mexican long-nosed -

Bioacoustics: the International Journal of Animal Sound and Its
This article was downloaded by: [National Science Library] On: 31 March 2015, At: 22:44 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Bioacoustics: The International Journal of Animal Sound and its Recording Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/tbio20 A comparative study in birds: call-type- independent species and individual recognition using four machine-learning methods and two acoustic features Jinkui Cheng a , Bengui Xie a , Congtian Lin a & Liqiang Ji a a Key Laboratory of Animal Ecology and Conservation Biology , Institute of Zoology, Chinese Academy of Sciences , 1-5 Beichenxi Road, Beijing , 100101 , China Published online: 26 Mar 2012. To cite this article: Jinkui Cheng , Bengui Xie , Congtian Lin & Liqiang Ji (2012) A comparative study in birds: call-type-independent species and individual recognition using four machine-learning methods and two acoustic features, Bioacoustics: The International Journal of Animal Sound and its Recording, 21:2, 157-171, DOI: 10.1080/09524622.2012.669664 To link to this article: http://dx.doi.org/10.1080/09524622.2012.669664 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. -

Herefore Takes Precedence
Introduction I have endeavored to keep typos, errors etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2011 to: [email protected]. Grateful thanks to Dick Coombes for the cover images. Joe Hobbs Index The general order of species follows the International Ornithological Congress’ World Bird List. Version Version 1.9 (1 August 2011). Cover Main image: Arctic Warbler. Cotter’s Garden, Cape Clear Island, Co. Cork, Ireland. 9 October 2009. Richard H. Coombes. Vignette: Arctic Warbler. The Waist, Cape Clear Island, Co. Cork, Ireland. 10 October 2009. Richard H. Coombes. Species Page No. Alpine Leaf Warbler [Phylloscopus occisinensis] 17 Arctic Warbler [Phylloscopus borealis] 24 Ashy-throated Warbler [Phylloscopus maculipennis] 20 Black-capped Woodland Warbler [Phylloscopus herberti] 5 Blyth’s Leaf Warbler [Phylloscopus reguloides] 31 Brooks’ Leaf Warbler [Phylloscopus subviridis] 22 Brown Woodland Warbler [Phylloscopus umbrovirens] 5 Buff-barred Warbler [Phylloscopus pulcher] 19 Buff-throated Warbler [Phylloscopus subaffinis] 17 Canary Islands Chiffchaff [Phylloscopus canariensis] 12 Chiffchaff [Phylloscopus collybita] 8 Chinese Leaf Warbler [Phylloscopus yunnanensis] 20 Claudia’s Leaf Warbler [Phylloscopus claudiae] 31 Davison’s Leaf Warbler [Phylloscopus davisoni] 32 Dusky Warbler [Phylloscopus fuscatus] 15 Eastern Bonelli's Warbler [Phylloscopus orientalis] 14 Eastern Crowned Warbler [Phylloscopus coronatus] 30 Emei Leaf Warbler [Phylloscopus emeiensis] 32 Gansu Leaf Warbler