Testing Ph Lab
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Fiber Optic Ammonia Sensor Using a Universal Ph Indicator
Sensors 2014, 14, 4060-4073; doi:10.3390/s140304060 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article A Fiber Optic Ammonia Sensor Using a Universal pH Indicator Adolfo J. Rodríguez 1,*, Carlos R. Zamarreño 2, Ignacio R. Matías 2, Francisco. J. Arregui 2, Rene F. Domínguez Cruz 1 and Daniel. A. May-Arrioja 1 1 Fiber and Integrated Optics Laboratory, Electronics Engineering Department, UAM Reynosa Rodhe, Universidad Autónoma de Tamaulipas, Carr. Reynosa-San Fernando S/N, Reynosa, Tamaulipas 88779, Mexico; E-Mails: [email protected] (R.F.D.C.); [email protected] (D.A.M.-A.) 2 Departamento de Ingeniería Eléctrica y Electrónica, Universidad Pública de Navarra, Edif. Los Tejos, Campus Arrosadia, Pamplona España 31006, Spain; E-Mails: [email protected] (C.R.Z.); [email protected] (I.R.M.); [email protected] (F.J.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +52-899-9213300 (ext. 8315); Fax: +52-899-921-3300 (ext. 8050). Received: 27 December 2013; in revised form: 12 February 2014 / Accepted: 18 February 2014 / Published: 27 February 2014 Abstract: A universal pH indicator is used to fabricate a fiber optic ammonia sensor. The advantage of this pH indicator is that it exhibits sensitivity to ammonia over a broad wavelength range. This provides a differential response, with a valley around 500 nm and a peak around 650 nm, which allows us to perform ratiometric measurements. The ratiometric measurements provide not only an enhanced signal, but can also eliminate any external disturbance due to humidity or temperature fluctuations. -

Properties of Acids and Bases
GREEN CHEMISTRY LABORATORY MANUAL Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? hat do you taste when you brush your teeth and drink orange juice afterwards. Yuck! It leaves a really bad taste in your mouth, but why? Orange juice and toothpaste by themselves taste good. But the terrible taste W results because an acid/base reaction is going on in your mouth. Orange juice is a weak acid and the toothpaste is a weak base. When they are placed together they neutralize each other and produce a product that is unpleasant to taste. How do you determine what is an acid and what is a base? In this lab we will discover how to distinguish between acids and bases. Introduction Two very important classes of compounds are acids and bases. But what exactly makes them different? There are differences in definition, physical differences, and reaction differences. According to the Arrhenius definition, acids ionize in water to + produce a hydronium ion (H3O ), and bases dissociate in water to produce hydroxide ion (OH -). Physical differences can be detected by the senses, including taste and touch. Acids have a sour or tart taste and can produce a stinging sensation to broken skin. For example, if you have ever tasted a lemon, it can often result in a sour face. Bases have a bitter taste and a slippery feel. Soap and many cleaning products are bases. -

Q1.Sodium Carbonate Reacts with Dilute Hydrochloric Acid
Q1.Sodium carbonate reacts with dilute hydrochloric acid: Na2CO3 + 2HCl → 2NaCl + H2O + CO2 A student investigated the volume of carbon dioxide produced when different masses of sodium car- bonate were reacted with dilute hydrochloric acid. This is the method used. 1. Place a known mass of sodium carbonate in a conical flask. 2. Measure 10 cm3 of dilute hydrochloric acid using a measuring cylinder. 3. Pour the acid into the conical flask. 4. Place a bung in the flask and collect the gas until the reaction is complete. (a) The student set up the apparatus as shown in the figure below. Identify the error in the way the student set up the apparatus. Describe what would happen if the student used the apparatus shown. (2) (b) The student corrected the error. The student’s results are shown in the table below. Mass of sodium carbonate Volume of carbon dioxide gas 3 in g in cm 0.07 16.0 0.12 27.5 0.23 52.0 0.29 12.5 0.34 77.0 0.54 95.0 0.59 95.0 0.65 95.0 The result for 0.29 g of sodium carbonate is anomalous. Suggest what may have happened to cause this anomalous result. (1) (c) Why does the volume of carbon dioxide collected stop increasing at 95.0 cm3? (1) (d) What further work could the student do to be more certain about the minimum mass of sodium M1.(a) (delivery) tube sticks into the acid 1 the acid would go into the water or the acid would leave the flask or go up the delivery tube ignore no gas collected 1 (b) any one from: • bung not put in firmly / properly • gas lost before bung put in • leak from tube 1 (c) all of the acid has reacted 1 (d) take more readings in range 0.34 g to 0.54 g 1 take more readings is insufficient ignore repeat 1 (e) The carbon dioxide was collected at room temperature and pressure. -

Ph Indicators Analysis Within Seconds Ph Indicator Strips
pH Indicators Analysis within Seconds pH Indicator Strips Economic in price In practice, it is normally quite sufficient to be able to measure pH in full units or in tenths of a unit. For this type of determination, as carried out in many laboratories, our various types of indicator paper, strips and liquids have proven themselves over many years. pH indicator paper has been on the market for decades in booklet and roll form. However, these forms are being more and more replaced by the more modern strip form (see next page). Indicator paper consists of high quality filter paper impregnated with indicator or indicator mixture. Order No. Designation pH range Graduation Roll length/ The table alongside shows the (*transition range) (pH units) No. of strips various types of booklet and rolls available Rolls 1.09565.0001 pH box 0.5 -13.0 0.5 3 x 4.8 m 1.09568.0001 Refill rolls, pH 0.5-5.0 0.5 - 5.0 0.5 6 x 4.8 m 1.09569.0001 Refill rolls, pH 5.5-9.0 5.5 - 9.0 0.5 6 x 4.8 m 1.09570.0001 Refill rolls, pH 9.5-13.0 9.5 -13.0 0.5 6 x 4.8 m 1.10962.0001 Universal indicator 1 -14.0 1 6 x 4.8 m 1.10232.0001 Refill rolls 6 x 4.8 m 1.09526.0001 Universal indicator 1 -10.0 1 6 x 4.8 m 1.09527.0001 Refill rolls 6 x 4.8 m 1.09560.0001 Acilit 0.5 - 5.0 0.5 6 x 4.8 m 1.09568.0001 Refill rolls 6 x 4.8 m 1.09564.0001 Neutralit 5.5 - 9.0 0.5 6 x 4.8 m 1.09569.0001 Refill rolls 6 x 4.8 m 1.09562.0001 Alkalit 9.5 -13.0 0.5 6 x 4.8 m 1.09570.0001 Refill rolls 6 x 4.8 m 1.09486.0001 Litmus paper, blue pH <7 red / >7 blue* 6 x 4.8 m 1.09489.0001 Litmus paper, red -

Detergent Residue Testing Using a Ph Meter, Ph Indicator, Or Test Kit
Alconox, Inc. The Leader in Critical Cleaning 30 Glenn St. White Plains NY 10603 USA Tel 914.948.4040 Fax 914-948-4088 www.alconox.com [email protected] Detergent Residue Testing Using a pH Meter, pH indicator, or Test Kit The following test procedures are suitable for detecting detergent residues resulting from improper rinsing and can be used to meet laboratory accreditation guidelines and questionnaires such as the College of American Pathologist program of State water lab accreditation programs. A. pH Meter Method 1. Rinse a small clean beaker by filling and emptying 3 times with source water. 2. Fill a 4th time and measure pH using a pH meter. Record the pH as source water pH. 3. Using a piece of cleaned glassware you wish to test, fill about 10% full with source water (10ml into 100ml beaker). Use more water if necessary to get enough water to be able to sufficiently immerse the pH meter electrode in your measuring beaker. 4. Swish water in glassware to extract residues from all possible surfaces. 5. Take pH reading with pH meter and record as glassware pH. 6. Any significant increase in pH indicates possible alkaline detergent residue. A significant change is 0.2 or more pH units on a pH meter measuring to 0.1 pH units of sensitivity. A result of less than 0.2-pH units change indicates properly rinsed glassware. Note: If deionized water is used as the sample water, a slight amount (10-20 mg/L) of reagent grade, non-buffering salt (NaCl, CaCl2) should be added to the sample water to allow pH meter to function properly. -
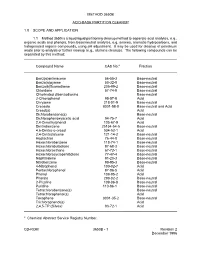
Method 3650B: Acid-Base Partition Cleanup, Part of Test Methods For
METHOD 3650B ACID-BASE PARTITION CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3650 is a liquid-liquid partitioning cleanup method to separate acid analytes, e.g., organic acids and phenols, from base/neutral analytes, e.g. amines, aromatic hydrocarbons, and halogenated organic compounds, using pH adjustment. It may be used for cleanup of petroleum waste prior to analysis or further cleanup (e.g., alumina cleanup). The following compounds can be separated by this method: _______________________________________________________________________________ Compound Name CAS No.a Fraction Benz(a)anthracene 56-55-3 Base-neutral Benzo(a)pyrene 50-32-8 Base-neutral Benzo(b)fluoranthene 205-99-2 Base-neutral Chlordane 57-74-9 Base-neutral Chlorinated dibenzodioxins Base-neutral 2-Chlorophenol 95-57-8 Acid Chrysene 218-01-9 Base-neutral Creosote 8001-58-9 Base-neutral and Acid Cresol(s) Acid Dichlorobenzene(s) Base-neutral Dichlorophenoxyacetic acid 94-75-7 Acid 2,4-Dimethylphenol 105-67-9 Acid Dinitrobenzene 25154-54-5 Base-neutral 4,6-Dinitro-o-cresol 534-52-1 Acid 2,4-Dinitrotoluene 121-14-2 Base-neutral Heptachlor 76-44-8 Base-neutral Hexachlorobenzene 118-74-1 Base-neutral Hexachlorobutadiene 87-68-3 Base-neutral Hexachloroethane 67-72-1 Base-neutral Hexachlorocyclopentadiene 77-47-4 Base-neutral Naphthalene 91-20-3 Base-neutral Nitrobenzene 98-95-3 Base-neutral 4-Nitrophenol 100-02-7 Acid Pentachlorophenol 87-86-5 Acid Phenol 108-95-2 Acid Phorate 298-02-2 Base-neutral 2-Picoline 109-06-8 Base-neutral Pyridine 110-86-1 Base-neutral Tetrachlorobenzene(s) Base-neutral Tetrachlorophenol(s) Acid Toxaphene 8001-35-2 Base-neutral Trichlorophenol(s) Acid 2,4,5-TP (Silvex) 93-72-1 Acid _______________________________________________________________________________ a Chemical Abstract Service Registry Number. -

Acids and Bases Experiments (Organic Classes)
Acids and Bases Experiments (Organic Classes) NaHCO3 (sodium bicarbonate, baking soda) and vinegar (dilute acetic acid) will be used to shoot a cork out of a bottle to show that some acid-base reactions generate gases like CO2. All students in the class will participate in a voice-activated chemical reaction. Students will remove a stopper and speak into a flask containing base and an indicator that will change color once enough CO2 is introduced from the students’ breath. Further color changes from basic to acidic conditions are illustrated using natural and household liquids and a universal indicator prepared from red cabbage juice. Finally, conversion between colorless and colored forms of different acid-base indicators in unusual settings will be shown in the “invisible painting” experiment. Supplies Needed: For the rocket reaction: You provide the following items indicated in red and starred: * empty 1 L plastic soda bottle with label removed * vinegar (colorless) Plastic powder funnel (we provide) cork with streamers attached by a thumbtack (we provide) container of de-ionized water (we provide, you refill as needed) sodium bicarbonate (we provide, return unused) Please clean and return all materials For the voice-activated chemical reaction: water that we provide as 250 mL Erlenmeyer flask (we provide)) soon as possible. dropper bottle with colorless phenolphtalein indicator (we provide) Other students may small amount of 1 M NaOH solution (we provide) need to use this “kit” disposable plastic droppers (we provide) for their ChemDemo!! For the universal pH indicator from red cabbage: * Red cabbage juice (you make the day before the demo) * 1 lemon, 1 soap bar, Sprite, vinegar, drain cleaner * 7 stirring rods (e.g. -

Linda Seppanen Garvin Heights Vineyards 2255 Garvin Heights Road Winona, MN
Linda Seppanen Garvin Heights Vineyards 2255 Garvin Heights Road Winona, MN Overview of winemaking Quality fruit Grapes are fermented by yeast and converted into wine. Winemaking procedure(s) differs at winemaker, winery, region, and country level. Many different techniques, recipes, outcomes. Desired wine style dictates much of winemaking techniques employed. Money, time and workers also important. Why we bother! Evaluating Wine –Objective Qualities Varietal character How well a wine presents the aromas and flavors inherent to the grapes from which it was made Integration How well all the components of wine are balanced and complementary to each other Expressiveness Well‐defined and clearly projected aromas and flavors Complexity That indescribable something that makes wine more art than beverage Connectedness The cultural connection a wine has to the place it was grown Components of Wine Alcohol Comes from fermentation; affects body, texture, aroma, & flavor May be sensed as a “hot” smell or burning sensation in the nose Acidity Comes from natural acid in the grape; may be sensed as tartness Wines lacking acidity taste dull, flat or flabby and do not age well Tannin Comes from seeds, skins and stems; adds “backbone” and “character” to the wine; is a natural preservative In overabundance, wine tastes harsh or bitter Fruitiness Propensity of wine to display fruity aromas and flavors Sugar (sweetness/dryness) Depends on how much of the grape’s original sugar content was converted to alcohol Not the same as fruitiness! Evaluating Wine Smell Taste Sight Evaluating Wine ‐ Smell Much of taste is smell, so getting a good whiff is important Aerate the wine by swirling it in the glass Stick your nose in the glass and inhale Called the nose, aroma, or bouquet Aroma traditionally refers to grape‐associated smells Bouquet refers to other smells (e.g. -

Estimating Ph at the Air/Water Interface with a Confocal
ANALYTICAL SCIENCES OCTOBER 2015, VOL. 31 1 2015 © The Japan Society for Analytical Chemistry Supporting Information Estimating pH at the Air/Water Interface with a Confocal Fluorescence Microscope Haiya YANG, Yasushi IMANISHI, Akira HARATA† Department of Molecular and Material Sciences, Interdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1 Kasugakoen, Kasuga-shi, Fukuoka 816-8580, Japan † To whom correspondence should be addressed. Email: [email protected] 1 2 ANALYTICAL SCIENCES OCTOBER 2015, VOL. 31 Mathematical relationship between fluorescence peak wavenumbers and pH for a fluorescent pH indicator In a confocal fluorescence microscope, the probe volume is confined in an elongated cylindrical shape with radius and height . If the position of the surface is defined to be exactly at the symmetrical plane horizontally intersecting the cylinder, the probe area and probe volume are and for the surface observation, while they are zero and for the bulk observation, respectively. For a component i, the ratio of fluorescent intensity detected for the surface observation with respect to the bulk observation can be given as (S1) where and represent the efficiencies of fluorescence excitation detection per fluorescent molecule at the surface and in the bulk solution, respectively; is the surface density, and is the bulk concentration. Because , Eq. (S1) is deformed into ; (S2) when , a surface-selective observation for this surface-active component at the water surface is available.17 In this case, and at a low concentration limit, the pH-dependent fluorescence spectrum of the surface-adsorbed pH indictor is given by , (S3) 2 ANALYTICAL SCIENCES OCTOBER 2015, VOL. -

Ph (Colorimetric) See a Separate Application for the Arena Or Gallery Analyzer
Page 1 D10493_03_Insert_pH_Colorimetric EN TEST PROCEDURE pH (colorimetric) See a separate application for the Arena or Gallery analyzer. 984349 Materials required but not provided 3 x 20 ml Reagent 1 Distilled water (aseptic and free of heavy metals) and general laboratory equipment. INTENDED USE Reagent for photometric determination of pH (colorimetric) in Calibrators (pH 3, 4, 5, 6). homogenous liquid samples using automated Thermo Scientific Arena pH 4 Std for QC, Thermo Fisher Scientific Cat no 984331 or Gallery analyzer. pH 7 Std for QC, Thermo Fisher Scientific Cat no 984332 METHOD Calibration Colorimetric test with pH indicator dyes in an aqueous solution. This method has been developed using 4 separate calibration points. Method is performed at 37 °C, using 575 nm filter and 700 nm as side Calibrators used were: wavelenght. Fixanal pH 3, Fixanal pH 4, Fixanal pH 5 and Fixanal pH 6 from PRINCIPLE OF THE PROCEDURE Sigma-Aldrich. pH (colorimetric) method is based on the property of acid-base indicator dyes, which produce color depending on the pH of the Note: Samples measured with manual pH meter can also be used as sample. The color change can be measured as an absorbance calibrators. In this case the calibration must be performed with same change spectrophotometrically. matrix type and with several points covering the whole measuring range. This calibration type must be validated by the user. REAGENT INFORMATION Barcode ID Quality Control Reagent 1 (R1) 3 x 20 ml A13 Use quality control samples at least once a day and after each calibration and every time a new bottle of reagent is used. -

Turning Image Sensors Into Position and Time Sensitive Quantitative Colorimetric Data Sources with the Aid of Novel Image Processing/Analysis Software
sensors Article Turning Image Sensors into Position and Time Sensitive Quantitative Colorimetric Data Sources with the Aid of Novel Image Processing/Analysis Software Yeongsik Yoo 1 and Woo Sik Yoo 2,* 1 College of Liberal Arts, Dankook University, Yongin-si 16890, Gyeonggi-do, Korea; [email protected] 2 WaferMasters, Dublin, CA 94568, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 9 November 2020; Published: 10 November 2020 Abstract: Still images and video images acquired from image sensors are very valuable sources of information. From still images, position-sensitive, quantitative intensity, or colorimetric information can be obtained. Video images made of a time series of still images can provide time-dependent, quantitative intensity, or colorimetric information in addition to the position-sensitive information from a single still image. With the aid of novel image processing/analysis software, extraction of position- and time-sensitive quantitative colorimetric information was demonstrated from still image and video images of litmus test strips for pH tests of solutions. Visual inspection of the color change in the litmus test strips after chemical reaction with chemical solutions is typically exercised. Visual comparison of the color of the test solution with a standard color chart provides an approximate pH value to the nearest whole number. Accurate colorimetric quantification and dynamic analysis of chemical properties from captured still images and recorded video images of test solutions using novel image processing/analysis software are proposed with experimental feasibility study results towards value-added image sensor applications. Position- and time-sensitive quantitative colorimetric measurements and analysis examples are demonstrated. -
![I24 [No Document #]](https://docslib.b-cdn.net/cover/4696/i24-no-document-1474696.webp)
I24 [No Document #]
Petrographic Analysis of Concrete Core Samples from Saltstone Disposal Unit #6 at the Savannah River Site, Aiken, SC Robert D. Moser, E. Rae Gore, and Kyle L. Klaus March 2016 Laboratory Geotechnical and Structures and Geotechnical i Executive Summary: This study examined five concrete cores provided to the ERDC by the U.S. Department of Energy Savannah River Site from its Saltstone Disposal Unit #6 facility (SDU6). The five cores, which were logged in as CMB No. 160051-1 to 160051-5 which were removed from three different floor placement locations in the structure were subjected to an in-depth analysis consisting of visual and petrographic examination, electron microscopy, pH measurements, elemental mapping, and air void analysis. The results of the study indicated through depth cracks in the slabs that appeared to be driven by shrinkage. Additional analysis identified that smaller cracks less than 0.1 mm in width possible occurring at early ages in the concrete had largely self healed while larger through depth cracks with widths greater than 0.1 mm and in many cases greater than 0.5 mm remained. Many of these large cracks had been successfully infilled with epoxy with the exception of large voids which intersected the cracks. Cracks often intersected coarse aggregates, indicating that the cracks likely occurred at later ages when the concrete strength was high. No vertical displacement was observed between adjacent crack faces. pH indicator solution measurements made using optical microscopy indicated that high pH remains within the concrete even adjacent to cracks. The near surface concrete has reduced pH to depths of up to 2 mm caused by exposure to acidic water and carbonation.