Feature Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Miniaturized Spectrophotometric in Situ Ph Sensor for Seawater
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2017 A miniaturized spectrophotometric in situ pH sensor for seawater Reuben C. Darlington The University Of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Part of the Chemical Engineering Commons, Environmental Chemistry Commons, Geochemistry Commons, and the Mechanical Engineering Commons Let us know how access to this document benefits ou.y Recommended Citation Darlington, Reuben C., "A miniaturized spectrophotometric in situ pH sensor for seawater" (2017). Graduate Student Theses, Dissertations, & Professional Papers. 11028. https://scholarworks.umt.edu/etd/11028 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. A MINIATURIZED SPECTROPHOTOMETRIC IN SITU pH SENSOR FOR SEAWATER By REUBEN CURTIS DARLINGTON B.A. Physics, The University of Montana, Missoula, MT, 2002 Thesis presented in partial fulfillment of the requirements for the degree of Master of Interdisciplinary Studies The University of Montana Missoula, MT Official Graduation Date Spring 2017 Approved by: Scott Whittenburg, Dean of The Graduate School Graduate School Michael D. DeGrandpre, Chair Chemistry J.B.A. Sandy Ross Chemistry Bradley E. Layton Applied Computing and Engineering Technology James C. Beck Sunburst Sensors, LLC Darlington, Reuben, M.I.S, Spring 2017 A Miniaturized Spectrophotometric in situ pH Sensor for Seawater Chairperson: Michael D. DeGrandpre Since the Industrial Revolution, the world's oceans have absorbed increasing amounts of CO2 and the resultant changes to the marine carbonate chemical system have reduced the pH by > 0.1 units (∼ 30%) in surface waters. -

Measuring Soil Ph
Measuring soil pH Viti-note Summary: Soil pH refers to the acidity or alkalinity Equipment of the soil. It is a measure of the • Equipment Colorimetric test kit available from concentration of free hydrogen ions nurseries (includes mixing stick, plate, • Timing (H+) that are in the soil. Soil pH can dye, barium sulphate, pH colour chart, be measured in water (pH ) or a weak • Method w instructions), teaspoon, recording sheet calcium chloride solution (pH ). The pH CaCl and pen. • Timing range is from 0-14, with value of 7 being neutral. Soil pH values (as measured in a OR water and soil solution) indicate: Hand held pH meter, clear plastic jar with • Strong acidity if less than 5.0. screw-on lid, distilled water, recording sheet and pen. • Moderate acidity at 5.0 to 6.0. • Neutral between 6.5 and 7.5. Timing • Moderate alkalinity at 7.5 to 8.5. This measurement is best undertaken • Strong alkalinity for values of 8.5 when soil sampling is conducted and and above. would normally be done at the same time The limited data available suggests that as assessments for electrical conductivity. soil pHCaCl should be in the range 5.5-7.5 Soil pH should be measured in the fibrous for best vine performance. root zone (ie. 0-20cm depth) as well as Soil pH outside the neutral range can the deeper root zone (>20cm depth). influence the availability of specific Make sure the soil samples are taken nutrients to plants, as well as the inside the irrigation wetting pattern. -

The Use Op the Glass Electrode As a Reference Electrode
THE USE OP THE GLASS ELECTRODE AS A REFERENCE ELECTRODE PAUL Si BROOKS ' Thesis submitted to the Faculty of the Graduate School of the University of Maryland in partial fulfillment of the requirements for the degree of Doctor of Philosophy 1940 UMI Number: DP70081 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMI Dissertation Publishing UMI DP70081 Published by ProQuest LLC (2015). Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code ProQuest ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Ac knowle dgement The writer takes this opportunity to express his appreciation to Dr. M. M* Haring for his suggestion of the problem and his continual aid during its completion. TABLE OP CONTENTS Page I. INTRODUCTION.......................... 1 II. THEORETICAL DISCUSSION . ......................................................................... 2 Theory of the Glass E lectro d e ..................................... 2 Standard Electrode Potential .............. 5 Application of the Glass Electrode ...................................... 10 III. EXPERIMENTAL.................................................................................................................... -

A Fiber Optic Ammonia Sensor Using a Universal Ph Indicator
Sensors 2014, 14, 4060-4073; doi:10.3390/s140304060 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article A Fiber Optic Ammonia Sensor Using a Universal pH Indicator Adolfo J. Rodríguez 1,*, Carlos R. Zamarreño 2, Ignacio R. Matías 2, Francisco. J. Arregui 2, Rene F. Domínguez Cruz 1 and Daniel. A. May-Arrioja 1 1 Fiber and Integrated Optics Laboratory, Electronics Engineering Department, UAM Reynosa Rodhe, Universidad Autónoma de Tamaulipas, Carr. Reynosa-San Fernando S/N, Reynosa, Tamaulipas 88779, Mexico; E-Mails: [email protected] (R.F.D.C.); [email protected] (D.A.M.-A.) 2 Departamento de Ingeniería Eléctrica y Electrónica, Universidad Pública de Navarra, Edif. Los Tejos, Campus Arrosadia, Pamplona España 31006, Spain; E-Mails: [email protected] (C.R.Z.); [email protected] (I.R.M.); [email protected] (F.J.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +52-899-9213300 (ext. 8315); Fax: +52-899-921-3300 (ext. 8050). Received: 27 December 2013; in revised form: 12 February 2014 / Accepted: 18 February 2014 / Published: 27 February 2014 Abstract: A universal pH indicator is used to fabricate a fiber optic ammonia sensor. The advantage of this pH indicator is that it exhibits sensitivity to ammonia over a broad wavelength range. This provides a differential response, with a valley around 500 nm and a peak around 650 nm, which allows us to perform ratiometric measurements. The ratiometric measurements provide not only an enhanced signal, but can also eliminate any external disturbance due to humidity or temperature fluctuations. -

Ph Meter Guide Ph Is a Measurement of Acidity Or Alkalinity in a Food Using a Numerical Scale from 1 to 14
FACT SHEET #31 pH Meter Guide pH is a measurement of acidity or alkalinity in a food using a numerical scale from 1 to 14. A pH below 7 is acidic, a pH of 7 is neutral, and a pH value above 7 is basic or alkaline. Monitoring pH levels during food processing is an important step in the production of some food since pH values affect microbial growth. Acidity and pH • Electrode: It is the part of the pH meter immersed in the sample. Select an electrode suitable for the food The acidity of food can be determined by measuring you are testing. For instance, some electrodes have its pH value. To preserve food with only acidity, it spear tips that are more suitable for measuring the pH needs to have an equilibrium pH value of 4.6 or lower. of semi-solid food. Equilibrium pH is the pH of a food after all components Use: of the food have achieved the same acidity. • Bench top models are suitable for laboratory use. If the pH meter will be taken into the plant, then a Foods with a pH greater than 4.6 are considered low handheld model may be more appropriate. acid foods. Foods with a pH less than 4.6 can be called acid foods. An acidified food is a low acid food Temperature with acidic ingredients added to it to lower the pH (ex: vinegar). Temperature can affect pH readings. To get an accurate reading, the pH meter must be calibrated at the same pH and micro-organisms temperature as the samples being tested. -

Developing a Non-Glass Ph Meter John Lyon Dr Raouf Selim
Developing a Non-Glass pH Meter John Lyon Dr Raouf Selim PCSE 498 Physics Capstone Project Fall 2014 - Spring 2015 Abstract I intended to develop and test a non-glass pH meter using an ion-selective field effect transistor (ISFET) for this project. The meter would have consisted of the ISFET, a reference electrode, and a voltmeter. The measurements made by this meter should agree with the Nernst equation which relates pH to potential difference. Unfortunately, materials for this project were very hard to come by, most notably, the ISFET itself. In lieu of this situation, I built a glass pH electrode instead. I used a test tube, potassium chloride, and silver wire to make the glass electrode. Upon building this meter, I found that the readings produced were very random and gave no conclusive results on how well the pH meter worked. Introduction I am interested in measuring the pH of a solution over a prolonged period of time. I decided that a non-glass pH meter would be appropriate because it does not contain a liquid solution within it. After some research about pH meters, I discovered that all potentiometric pH meters have a reference electrode, and generally these electrodes contain a reference solution, so my non-glass meter might still require this reference solution. Because I did not want to have an internal solution in my pH meter, I thought it would be interesting to develop a simulated reference electrode along with the non-glass electrode. The non-glass electrode to be used would be an ion-selective field effect transistor (ISFET) electrode. -

Detergent Residue Testing Using a Ph Meter, Ph Indicator, Or Test Kit
Alconox, Inc. The Leader in Critical Cleaning 30 Glenn St. White Plains NY 10603 USA Tel 914.948.4040 Fax 914-948-4088 www.alconox.com [email protected] Detergent Residue Testing Using a pH Meter, pH indicator, or Test Kit The following test procedures are suitable for detecting detergent residues resulting from improper rinsing and can be used to meet laboratory accreditation guidelines and questionnaires such as the College of American Pathologist program of State water lab accreditation programs. A. pH Meter Method 1. Rinse a small clean beaker by filling and emptying 3 times with source water. 2. Fill a 4th time and measure pH using a pH meter. Record the pH as source water pH. 3. Using a piece of cleaned glassware you wish to test, fill about 10% full with source water (10ml into 100ml beaker). Use more water if necessary to get enough water to be able to sufficiently immerse the pH meter electrode in your measuring beaker. 4. Swish water in glassware to extract residues from all possible surfaces. 5. Take pH reading with pH meter and record as glassware pH. 6. Any significant increase in pH indicates possible alkaline detergent residue. A significant change is 0.2 or more pH units on a pH meter measuring to 0.1 pH units of sensitivity. A result of less than 0.2-pH units change indicates properly rinsed glassware. Note: If deionized water is used as the sample water, a slight amount (10-20 mg/L) of reagent grade, non-buffering salt (NaCl, CaCl2) should be added to the sample water to allow pH meter to function properly. -

2105 Soilstik
® SoilStik pH Meter PRODUCT MANUAL Item # 2105 2 Contents General Overview .....................................3 LCD Display................................................4 LCD Display Messages ............................5 Meter Components ...................................6 Calibration .................................................7 Changing the Temperature Units.............8 Taking Liquid/Soil Measurements ...........9 Storing/Recalling Measurements...........10 Sensor Replacement ...............................11 Battery Replacement...............................12 Meter Handling/Troubleshooting ..........13 Specifications .........................................14 Warranty ...................................................15 This manual will familiarize you with the features and operation of your new meter. Please read this manual thoroughly before using your meter. For customer support or to place an order, contact Spectrum Technologies, Inc between 7:30 a.m. and 5:30 p.m. CST. Phone: (800) 248-8873 or (815) 436-4440 Fax: (815) 436-4460 E-mail: [email protected] Website: www.specmeters.com Spectrum Technologies 12360 S. Industrial Dr. East Plainfield, IL 60585 3 General Overview Congratulations on the purchase of your Field Scout™ SoilStik™ Pro Meter. This manual describes how to use your pH meter and keep it working accurately. Read the manual thoroughly in order to make effective use of your meter. The SoilStik delivers high quality answers, with an accuracy of +/- 0.01 pH units. This self-contained digital meter allows you to test the pH levels in water, soil, and other liquids. The replaceable sensor makes the measurement of small samples much more convenient. The sensor provides a visual indication of when replacement is due. The meter has a two-point, automatic calibration (pH 4 and 7), with a range of pH 2.0-12.0. The display will show your results to a resolution of 0.01 pH units. -
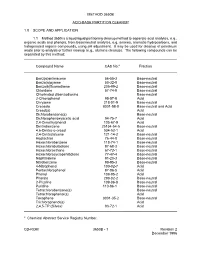
Method 3650B: Acid-Base Partition Cleanup, Part of Test Methods For
METHOD 3650B ACID-BASE PARTITION CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3650 is a liquid-liquid partitioning cleanup method to separate acid analytes, e.g., organic acids and phenols, from base/neutral analytes, e.g. amines, aromatic hydrocarbons, and halogenated organic compounds, using pH adjustment. It may be used for cleanup of petroleum waste prior to analysis or further cleanup (e.g., alumina cleanup). The following compounds can be separated by this method: _______________________________________________________________________________ Compound Name CAS No.a Fraction Benz(a)anthracene 56-55-3 Base-neutral Benzo(a)pyrene 50-32-8 Base-neutral Benzo(b)fluoranthene 205-99-2 Base-neutral Chlordane 57-74-9 Base-neutral Chlorinated dibenzodioxins Base-neutral 2-Chlorophenol 95-57-8 Acid Chrysene 218-01-9 Base-neutral Creosote 8001-58-9 Base-neutral and Acid Cresol(s) Acid Dichlorobenzene(s) Base-neutral Dichlorophenoxyacetic acid 94-75-7 Acid 2,4-Dimethylphenol 105-67-9 Acid Dinitrobenzene 25154-54-5 Base-neutral 4,6-Dinitro-o-cresol 534-52-1 Acid 2,4-Dinitrotoluene 121-14-2 Base-neutral Heptachlor 76-44-8 Base-neutral Hexachlorobenzene 118-74-1 Base-neutral Hexachlorobutadiene 87-68-3 Base-neutral Hexachloroethane 67-72-1 Base-neutral Hexachlorocyclopentadiene 77-47-4 Base-neutral Naphthalene 91-20-3 Base-neutral Nitrobenzene 98-95-3 Base-neutral 4-Nitrophenol 100-02-7 Acid Pentachlorophenol 87-86-5 Acid Phenol 108-95-2 Acid Phorate 298-02-2 Base-neutral 2-Picoline 109-06-8 Base-neutral Pyridine 110-86-1 Base-neutral Tetrachlorobenzene(s) Base-neutral Tetrachlorophenol(s) Acid Toxaphene 8001-35-2 Base-neutral Trichlorophenol(s) Acid 2,4,5-TP (Silvex) 93-72-1 Acid _______________________________________________________________________________ a Chemical Abstract Service Registry Number. -

Acids and Bases Experiments (Organic Classes)
Acids and Bases Experiments (Organic Classes) NaHCO3 (sodium bicarbonate, baking soda) and vinegar (dilute acetic acid) will be used to shoot a cork out of a bottle to show that some acid-base reactions generate gases like CO2. All students in the class will participate in a voice-activated chemical reaction. Students will remove a stopper and speak into a flask containing base and an indicator that will change color once enough CO2 is introduced from the students’ breath. Further color changes from basic to acidic conditions are illustrated using natural and household liquids and a universal indicator prepared from red cabbage juice. Finally, conversion between colorless and colored forms of different acid-base indicators in unusual settings will be shown in the “invisible painting” experiment. Supplies Needed: For the rocket reaction: You provide the following items indicated in red and starred: * empty 1 L plastic soda bottle with label removed * vinegar (colorless) Plastic powder funnel (we provide) cork with streamers attached by a thumbtack (we provide) container of de-ionized water (we provide, you refill as needed) sodium bicarbonate (we provide, return unused) Please clean and return all materials For the voice-activated chemical reaction: water that we provide as 250 mL Erlenmeyer flask (we provide)) soon as possible. dropper bottle with colorless phenolphtalein indicator (we provide) Other students may small amount of 1 M NaOH solution (we provide) need to use this “kit” disposable plastic droppers (we provide) for their ChemDemo!! For the universal pH indicator from red cabbage: * Red cabbage juice (you make the day before the demo) * 1 lemon, 1 soap bar, Sprite, vinegar, drain cleaner * 7 stirring rods (e.g. -

Aerospace Research Center
Aerospace Research Center BEAM ALIGNMENT TECHNIQUES BASED ON THE CURRENT ENHANCEMENT EFFECT IN PHOTOCONDUCTORS FI NAL TECHNICAL REPORT 3 1 August 1968. This document was prepared under the sponsorship of the 3 ! National Aeronautics & Space Administration. Neither .A the United States Government nor any person acting on behalf of the United States Government assumes any liability resulting from the use of information contained in this document, or warrants that such use wi I I be free from privately-owned rights. > Prepared under Contract NAS 12-8 by w GENERAL PRECISION SYSTEMS INC. Aerospace Research Center Little Falls, New Jersey for National Aeronautics and Space Administration Electronics Research Center Cambridge, Massachusetts Y Mr. Janis Bebris Technical Minitor EO/Space Optics Laboratory E Iectronics Research Center 575 Technology Square Cambridge, Massachusetts 02 139 .-. Requests for copies of this report should be referred to: NASA Scientific and Technical Information Facility, P.O. Box 33, College Park, Maryland 20740 AEROSPACE RESEARCH CENTER 0 GENERAL PRECISION SYSTEMS INC. RC -68 -8 BEAM ALIGNMENT TECHNIQUES BASED ON THE CURRENT ENHANCEMENT EFFECT IN PHOTOCONDUCTORS Work Performed By: Written By: Cecil B. Ellis Frank V. Allan Raymond P, Borkowski William M, Block Alfred Brauer Robert Carval ho Cecil B. Ellis Robert Flower Jesse C. Kaufman .. Aryeh H. Samuel li Joseph C, Scanlon Dr. Daniel Grirfstein Principal Staff Scientist Manager, Materials Department 31 August 1968 FINAL TECHNICAL REPORT Prepared under Contract NAS 12-8 by GENERAL PRECISION SYSTEMS INC. Aerospace Research Center Little Falls, New Jersey for the Electronics Research Center National Aeronautics and Space Administration V i AEROSPACE RESEARCH CENTER GENERAL PRECISION SYSTEMS INC. -

The 50 Series Ph Meter
The 50 Series pH Meter Takeshi Kobayashi [The Development Team (Main body)] Upper left: Yuichi Ito Back row from the left: Seiichiro Yoshioka, Takeshi Shimizu, Hiroyuki Kitamura, Takeshi Mori, Yosuke Hisamori Front row from the left: Yoshiyuki Okada, Mitsuru Honjo, Yoshihiro Tarui, Katsuaki Ogura, Yoshihiko Ida [The Development Team (Electrode)] Back row from the left: Yuji Nishio, Yasukazu Iwamoto, Nobuki Yoshioka, Shinji Takeichi, Takeshi Kobayashi, Kenichi Tanaka Front row from the left: Hiromi Ohkawa, Tsuyoshi Nakanishi, Satoshi Nomura, Hiroki Tanabe, Koji Ueda In developing the 50 Series, we focused on the following two points: ease of use of the basic pH meter main unit and enhancement of the electrode performance as the most vital features for pH measurement. By adding a navigation function with character display, which is not used by conventional analyzers, as well as adopting the world’s first color display, our new bench top pH meter has proved user friendly even for customers with no pH measurement knowledge. We took the World’s top performing ToupH glass electrode series and succeeded in increasing the electrode life by improving the performance of the reference electrode and strengthening the durability of glass material. Also, the pH measurement field has been extended to new and unconventional measurement areas by commercialization of the glass- free ISFET electrode. This article describes features of this product in detail. 88 English Edition No.9 Technical Reports Introduction pH Meter Main Unit This product is commercially manufactured as the 50 series This development has seen a total of 14 models of the D-50 and is a model change from the conventional D-20 series series for on-site measurement and also the F-50 series for and F-20 series and commemorates HORIBA’s 50th laboratory use, simultaneously produced in approximately anniversary.