A Unique Resource 1.2. World Water Distribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019060814.Pdf
DISTRICT SURVEY REPORT TIRUPPUR DISTRICT Chapter Content Page No. 1. Introduction 4 2. Overview of Mining Activity in the District 4 – 5 3. General Profile of the District 5 – 8 4. Geology of Tiruppur District 9 – 12 5. Drainage of Irrigation pattern 12 Land Utilisation Pattern in the District: Forest, Agricultural, 6. 13 – 14 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 14 – 15 8. Climate and Rainfall of the District 16 9. Details of Mining Leases in the District 17 10. Details of Royalty or Revenue received in last three years 18 11. Details of Production of Minor Mineral in last three years 19 12. Mineral Map of the District 20 List of Letter of Intent (LOI) Holder in the District along with 13. 21 its validity 14. Total Mineral Reserve available in the district 21 15. Quality/Grade of Mineral available in the district 22 16. Use of Mineral 22 17. Demand and supply of the Mineral in the last three years 22 18. Mining Leases marked on the map of the district 23 – 24 Details of the area of where there is a cluster of the mining 19. 25 leases 20. Details of Eco-sensitive area 25 21. Impact on the environment due to Mining activity 25 – 26 Remedial measures to mitigate the impact of mining on the 22. 27 – 28 environment 23. Reclamation of the mined out area 29 - 30 24. Risk assessment & Disaster Management Plan 30 – 31 25. Details of Occupational health issue in the District 31 – 32 Plantation and Green belt development in respect of leases 26. -

Seasonal Variation of Cauvery River Due to Discharged Industrial Effluents at Pallipalayam in Namakkal
Vol. 8 | No. 3 |380 - 388 | July - September | 2015 ISSN: 0974-1496 | e-ISSN: 0976-0083 | CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in SEASONAL VARIATION OF CAUVERY RIVER DUE TO DISCHARGED INDUSTRIAL EFFLUENTS AT PALLIPALAYAM IN NAMAKKAL K. Sneka Lata 1, A. Jesu 2, M.S. Dheenadayalan 1 1Department of Chemistry G.T.N. Arts College, Dindigul, Tamil Nadu. India. 2Department of Chemistry, Kathir College of Engineering, Neelambur, Coimbatore (T.N.)India *E-mail: [email protected] ABSTRACT The impact of industrial effluent like dyeing, sugar, and paper discharged from the banks of Cauvery river at pallipalayam in Namakkal district. It is observed during the study that many dyeing, sugar and paper units discharged their untreated effluent into the river Cauvery in this criminately without any treatment. The river water samples and ground water samples and soil sample collected in the study area reveals that high degree of the pollution cost by untreated effluent of heavy metal analysis from the river water and ground water and soil. So that industries major culprit in damaging the river water, ground water and soil used for the agricultural purpose. The increased loading of toxic effluent day by day due to the toxic effluent of surface water, ground water and soil. The total pollution due to industrial effluent causes the great damage to the environmental pollution of river Cauvery at pallipalayam in Namakkal district. Keywords: Raw effluents, treated effluents, total dissolved solids, dyeing industry, physico chemical analysis ©2015 RAS ĀYAN. All rights reserved INTRODUCTION The Kaveri, also spelled Cauvery in English, is a large Indian river. -

OCCASIO I AL PAPER O. 36 RECORDS of the ZOOLOGICAL SURVEY of INDIA
MISCELLANEOUS PUBLICATION OCCASIO I AL PAPER o. 36 RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA MISCELLANEOUS PUBLICATION OCCASIONAL PAPER No. 36 A SURVEY OF THE CAUVERY RIVER SYSTEM WITH A MAJOR ACCOUNT OF ITS FISH FAUNA BY K. C. Jayaram Zoological Survey C!! India, Oalcutta-700 016 AND T~ Venkateswarlu" M. B. Ragunathan S.kern Regional Station, Zoological Survey of India, Madras 600 028 Edited by the Director, Zoological Survey. of India 1982 ® Copyright 1982, Government of India Published in August, 1982 PRICE: 1 nlana : Rs. 4~.OO Foreign : £ 6.00 $ 9,50 PRINTED ~N INDIA BY THB BANI PRESS, 16 HBMENDRA SBN STRBBT, CALCUTTA-700 006 AND PUBLISHED BY THB DIRBCTOR, ZOOLOGICAL SURVBY OP INDIA, CALCUTTA. RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA Miscellaneous Publication Occasional Paper No. 36 1982 Pages 1-115 CONTENTS PAGE INTRODUCTION 1 WORK PROGRAMME ... 1 AUTHORSHIP ASSIGNMENTS 2 ACKNOWLEDGEMENTS 3 THE CAUVERY RIVER 3 CLIMATE AND VEGETATION 5 TRIBUTARIES 5 COLLECTING STATIONS WITH ECOLOGICAL NOTES 7 MARGINAL AND AQUATIC BIOTA 18 SYSTEMATIC LIST OF CAUVERY FISHES 20 SYSTEMATIC ACCOUNT ••• 28 DISCUSSION 107 CONCLUSIONS AND RECOMMENDATIONS 110 REFERENCES • • . , •• 112 INTRODUCTION Cauvery, Krishna and Godavary rivers constitute the major three ,1.er systems in South India. Geologically they are much older than die Oanga, Indus and Brahmaputra rivers of Northen India. The eco nomic prosperity of the southern states of Andhra Pradesh, Tamil Nadu Kerala and Karnataka is closely intertwined with the water-supply and potentialities of these three rivers. Since historical times their. waters have been extensively utilised for agriculture, fisheries, irrigation and tllYigation purposes. -

BHISHMA SRI. R. RADHAKRISHNAN Former President & Trustee - BAI 81St Birthday on 2Nd July 2021 Wishing a Happy Healthy Long Life
Southern Estd1941 Bulletin of Builders Association of India - Southern Centre JULY 2021 BuilderFor Private Circulation only BHISHMA SRI. R. RADHAKRISHNAN Former President & Trustee - BAI 81st Birthday On 2nd July 2021 Wishing a Happy healthy long life Southern Estd1941 Bulletin of Builders Association of India - Southern Centre BuilderFor Private Circulation only Offical Journal of Builders’ Association of India - Southern Centre. July- 2021 Builders’ Association of India Southern Centre Plot No. A1, 1st Main Road, Opp. to AIEMA, Industrial Estate, Ambattur, Chennai - 600 058. (T) 044-2625 2006 | (E) [email protected] | (W) www.baisouthern.com CONTENTS OFFICE BEARERS - 2020-2021 Mr. L SHANTHAKUMAR - Chairman Ýê¤ó¤ò£¢ ñìô¢ 04 Mr. R R SHRIDHAR - Vice Chairman ñò¢òî¢î¬ôõ£¢ ñìô¢ 05 Mr. A N BALAJI - Hon. Secretary Mr. R NIMRODE - Hon. Joint Secretary Structures in the context of Mr. N G LOKANATHAN - Hon. Treasurer 06 everyday life EDITOR Mr. S AYYANATHAN Rules for Enlistment of Contractors in 08 98410 46799 CPWD, 2021 EDITORIAL BOARD Income Tax Benefits for Senior Citizens 15 Mr. L VENKATESAN Mr. S D KANNAN Tax Corner 18 Mr. P K P NARAYANAN ð¦û¢ñ£ R. Þó£î£è¤¼û¢íù¢¢ 24 ADVISORS Üè¬õ "81" Bhisma. R RADHAKRISHNAN All India Past President & Trustee - BAI ºù¢«ù££¢è÷¢ õ°î¢î 40 õ¬è 30 ñ¬öè÷¢ Mr. Mu MOAHAN Imm. Past National President - BAI Southern Centre Activities 42 Mr. J R SETHURAMALINGAM All India Past Vice President & Trustee - BAI TARIFF Si. No. Description Rate Per Issue Rate Per Annum 1. Multi Colour A4 Size Rear Cover Outer Rs.30,000/- Rs.3,00,000/- 2. -

Problems of Salination of Land in Coastal Areas of India and Suitable Protection Measures
Government of India Ministry of Water Resources, River Development & Ganga Rejuvenation A report on Problems of Salination of Land in Coastal Areas of India and Suitable Protection Measures Hydrological Studies Organization Central Water Commission New Delhi July, 2017 'qffif ~ "1~~ cg'il'( ~ \jf"(>f 3mft1T Narendra Kumar \jf"(>f -«mur~' ;:rcft fctq;m 3tR 1'j1n WefOT q?II cl<l 3re2iM q;a:m ~0 315 ('G),~ '1cA ~ ~ tf~q, 1{ffit tf'(Chl '( 3TR. cfi. ~. ~ ~-110066 Chairman Government of India Central Water Commission & Ex-Officio Secretary to the Govt. of India Ministry of Water Resources, River Development and Ganga Rejuvenation Room No. 315 (S), Sewa Bhawan R. K. Puram, New Delhi-110066 FOREWORD Salinity is a significant challenge and poses risks to sustainable development of Coastal regions of India. If left unmanaged, salinity has serious implications for water quality, biodiversity, agricultural productivity, supply of water for critical human needs and industry and the longevity of infrastructure. The Coastal Salinity has become a persistent problem due to ingress of the sea water inland. This is the most significant environmental and economical challenge and needs immediate attention. The coastal areas are more susceptible as these are pockets of development in the country. Most of the trade happens in the coastal areas which lead to extensive migration in the coastal areas. This led to the depletion of the coastal fresh water resources. Digging more and more deeper wells has led to the ingress of sea water into the fresh water aquifers turning them saline. The rainfall patterns, water resources, geology/hydro-geology vary from region to region along the coastal belt. -

RTM-February -2020 Magazine
INSIGHTSIAS IA SIMPLIFYING IAS EXAM PREPARATION RTM COMPILATIONS PRELIMS 2020 FEBRUARY 2020 www.insightsactivelearn.com | www.insightsonindia.com Revision Through MCQs (RTM) Compilation (February 2020) Telegram: https://t.me/insightsIAStips 2 Youtube: https://www.youtube.com/channel/UCpoccbCX9GEIwaiIe4HLjwA Revision Through MCQs (RTM) Compilation (February 2020) Telegram: https://t.me/insightsIAStips 3 Youtube: https://www.youtube.com/channel/UCpoccbCX9GEIwaiIe4HLjwA Revision Through MCQs (RTM) Compilation (February 2020) Table of Contents RTM- REVISION THROUGH MCQS – 1st Feb-2020 ............................................................... 5 RTM- REVISION THROUGH MCQS – 3st Feb-2020 ............................................................. 10 RTM- REVISION THROUGH MCQS – 5th Feb-2020 ............................................................. 16 RTM- REVISION THROUGH MCQS – 6th Feb-2020 ............................................................. 22 RTM- REVISION THROUGH MCQS – 7th Feb-2020 ............................................................. 28 RTM- REVISION THROUGH MCQS – 8th Feb-2020 ............................................................. 34 RTM- REVISION THROUGH MCQS – 10th Feb-2020 ........................................................... 40 RTM- REVISION THROUGH MCQS – 11th Feb-2020 ........................................................... 45 RTM- REVISION THROUGH MCQS – 12th Feb-2020 ........................................................... 52 RTM- REVISION THROUGH MCQS – 13th Feb-2020 .......................................................... -

ROMANTIC SOUTH Starts at Bangalore Ends at Calicat
ROMANTIC SOUTH Starts At Bangalore Ends At Calicat Itinerary DAY 1 BANGALORE €“ MYSORE ( 150 KMS / 3 ½ HRS ) Pick up from Bangalore Airport and drive to Mysore on arrival check in to the hotel. Proceed for local sightseeing (if time permits) else day at leisure or own activities and overnight stay at Mysore. Mysore Mysore (or Mysuru), is second largest city in Karnataka state which covers an area of more than 40 sqkm and is administered by the Mysore City Corporation. Situated 763 meters above sea level surrounded by hill ranges from north to south, it is known as the ‘Garden City’ and the ‘City of Palaces’. It was the capital of the Kingdom of Mysore from 1399-1947. In its centre is opulent Mysore Palace, former seat of the ruling Wodeyar dynasty. The palace blends Hindu, Islamic, Gothic and Rajput styles, and is dramatically lit at night. Mysore is also famous for the centuries-old Devaraja Market, filled with spices, silk and sandalwood. Sight seeings in Mysore Mysore Sightseeing includes Mysore palace, St Philomina’s Church & Brindavan Garden DAY 2 MYSORE €“ COORG ( 110 KMS / 2 ½ HRS ) After breakfast checkout from hotel and drive to Coorg, on arrival check in to the hotel. Proceed for local Sightseeing (if time permits) else day at leisure or own activities and overnight stay at Coorg. Coorg Coorg, also known as Kodagu, by its anglicised former name of Coorg, is a mountainous district located in south of India, known for its beautiful scenery and hospitable people. Glorious sounds, sights and scents welcome you as you enter Coorg. -

Nagapattinam District 64
COASTAL DISTRICT PROFILES OF TAMIL NADU ENVIS CENTRE Department of Environment Government of Tamil Nadu Prepared by Suganthi Devadason Marine Research Institute No, 44, Beach Road, Tuticorin -628001 Sl.No Contents Page No 1. THIRUVALLUR DISTRICT 1 2. CHENNAI DISTRICT 16 3. KANCHIPURAM DISTRICT 28 4. VILLUPURAM DISTRICT 38 5. CUDDALORE DISTRICT 50 6. NAGAPATTINAM DISTRICT 64 7. THIRUVARUR DISTRICT 83 8. THANJAVUR DISTRICT 93 9. PUDUKOTTAI DISTRICT 109 10. RAMANATHAPURAM DISTRICT 123 11. THOOTHUKUDI DISTRICT 140 12. TIRUNELVELI DISTRICT 153 13. KANYAKUMARI DISTRICT 174 THIRUVALLUR DISTRICT THIRUVALLUR DISTRICT 1. Introduction district in the South, Vellore district in the West, Bay of Bengal in the East and i) Geographical location of the district Andhra Pradesh State in the North. The district spreads over an area of about 3422 Thiruvallur district, a newly formed Sq.km. district bifurcated from the erstwhile Chengalpattu district (on 1st January ii) Administrative profile (taluks / 1997), is located in the North Eastern part of villages) Tamil Nadu between 12°15' and 13°15' North and 79°15' and 80°20' East. The The following image shows the district is surrounded by Kancheepuram administrative profile of the district. Tiruvallur District Map iii) Meteorological information (rainfall / ii) Agriculture and horticulture (crops climate details) cultivated) The climate of the district is moderate The main occupation of the district is agriculture and allied activities. Nearly 47% neither too hot nor too cold but humidity is of the total work force is engaged in the considerable. Both the monsoons occur and agricultural sector. Around 86% of the total in summer heat is considerably mitigated in population is in rural areas engaged in the coastal areas by sea breeze. -
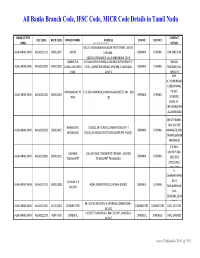
Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu
All Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu NAME OF THE CONTACT IFSC CODE MICR CODE BRANCH NAME ADDRESS CENTRE DISTRICT BANK www.Padasalai.Net DETAILS NO.19, PADMANABHA NAGAR FIRST STREET, ADYAR, ALLAHABAD BANK ALLA0211103 600010007 ADYAR CHENNAI - CHENNAI CHENNAI 044 24917036 600020,[email protected] AMBATTUR VIJAYALAKSHMIPURAM, 4A MURUGAPPA READY ST. BALRAJ, ALLAHABAD BANK ALLA0211909 600010012 VIJAYALAKSHMIPU EXTN., AMBATTUR VENKATAPURAM, TAMILNADU CHENNAI CHENNAI SHANKAR,044- RAM 600053 28546272 SHRI. N.CHANDRAMO ULEESWARAN, ANNANAGAR,CHE E-4, 3RD MAIN ROAD,ANNANAGAR (WEST),PIN - 600 PH NO : ALLAHABAD BANK ALLA0211042 600010004 CHENNAI CHENNAI NNAI 102 26263882, EMAIL ID : CHEANNA@CHE .ALLAHABADBA NK.CO.IN MR.ATHIRAMIL AKU K (CHIEF BANGALORE 1540/22,39 E-CROSS,22 MAIN ROAD,4TH T ALLAHABAD BANK ALLA0211819 560010005 CHENNAI CHENNAI MANAGER), MR. JAYANAGAR BLOCK,JAYANAGAR DIST-BANGLAORE,PIN- 560041 SWAINE(SENIOR MANAGER) C N RAVI, CHENNAI 144 GA ROAD,TONDIARPET CHENNAI - 600 081 MURTHY,044- ALLAHABAD BANK ALLA0211881 600010011 CHENNAI CHENNAI TONDIARPET TONDIARPET TAMILNADU 28522093 /28513081 / 28411083 S. SWAMINATHAN CHENNAI V P ,DR. K. ALLAHABAD BANK ALLA0211291 600010008 40/41,MOUNT ROAD,CHENNAI-600002 CHENNAI CHENNAI COLONY TAMINARASAN, 044- 28585641,2854 9262 98, MECRICAR ROAD, R.S.PURAM, COIMBATORE - ALLAHABAD BANK ALLA0210384 641010002 COIIMBATORE COIMBATORE COIMBOTORE 0422 2472333 641002 H1/H2 57 MAIN ROAD, RM COLONY , DINDIGUL- ALLAHABAD BANK ALLA0212319 NON MICR DINDIGUL DINDIGUL DINDIGUL -

Irrigation Projects of Tamil Nadu from 2001-2021
IRRIGATION PROJECTS OF TAMIL NADU FROM 2001-2021 NAME – VRINDA GUPTA INSTITUTION – K.R. MANGALAM UNIVERSITY 1 ABSTRACT From the ancient times water is always most important for agriculture purpose for growing crops. Since thousand years, humans have relied on agriculture to feed their communities and they have needed irrigation to water their crops. Irrigation includes artificially applying water to the land to enhance the growing of crops. Over the years, irrigation has come in many different forms in countries all over the world. Irrigation projects involves hydraulic structures which collect, convey and deliver water to those areas on which crops are grown. Irrigation projects unit may starts from a small farm unit to those serving extensive areas of millions of hectares. Irrigation projects consist of two types first a small irrigation project and second a large irrigation project. Small irrigation project includes a low diversion or an inexpensive pumping plant along with small channels and some minor control structures. Large irrigation project includes a huge dam, a large storage reservoir, hundreds kilometers of canals, branches and distributaries, control structures and other works. In this paper we discussing about irrigation plan of Tamil Nadu from 2001-2021. INTRODUCTION Water is the important or elixir of life, a precious gift of nature to humans and millions of other species living on the earth. It is hard to find in most part of the world. 4% of India’s land area in Tamil Nadu and inhabited by 6% of India’s population but water resources in India is only 2.5%. In Tamil Nadu, water is a serious limiting factor for agriculture growth which leads to irrigation reduces risk in farming, increases crop productivity, provides higher employment opportunities to the rural areas and increases farmer income. -

Time Series Analysis for Water Inflow
Published by : International Journal of Engineering Research & Technology (IJERT) http://www.ijert.org ISSN: 2278-0181 Vol. 6 Issue 11, November - 2017 Time Series Analysis for Water Inflow Sara Kutty T K* Hanumanthappa M Research Scholar, Computer Applications Department, Rayalaseema University Bangalore University Kurnool, India Bangalore, India Abstract—The main sources of water are natural sources like This paper aims at a time series analysis of Cauvery water in rainwater, oceans, rivers, lakes, streams, ponds, springs etc., and Karnataka. In particular time series of data is plotted to find man-made sources like dams, wells, tube wells, hand-pumps, any possible trend. Using ARIMA model future water inflows canals, etc. Agriculture and plantation depends heavily on water is plotted. We would like to use the data for water and its source is rainfall. Mathematical models are required to management analysis and for model based statistical inference predict future water demand and climate changes. Time series are valuable sources of information that can be consulted for the of environmental systems that account for the need of using characterization of variables in several areas of knowledge. It is prior information, input and model structure uncertainty. In basically a measurement of data taken in chronological order order to analyze the water inflow the average wet weather within a certain time. In hydrology, such series are employed in flow can be estimated from water flow data. Water inflow is water systems management as tools for the hydrological cycle defined as the water other than sanitary wastewater that enters understanding. The purpose of most water quality and stream a sewer system from sources such as roof leaders, flow studies is to point out the information and necessary cellar/foundation drains, yard drains, area drains, drains etc. -

Thiruchirappal Disaster Managem Iruchirappalli
Tiruchirappalli District Disaster Management Plan – 2020 THIRUCHIRAPPALLI DISTRICT DISASTER MANAGEMENT PLAN-2020 Tiruchirappalli District Disaster Management Plan – 2020 INDEX S. Particulars Page No. No. 1. Introduction 1 2. District Profile 2-4 3. Disaster Management Goals (2017-2030) 5-11 4. Hazard, Risk and Vulnerability Analysis with Maps 12-49 (District map, Division maps, Taluk maps & list of Vulnerable area) 5. Institutional Mechanism 50-52 6. Preparedness Measures 53-56 7. Prevention and Mitigation measures (2015 – 2030) 57-58 8. Response Plan 59 9. Recovery and Reconstruction Plan 60-61 10. Mainstreaming Disaster Management in Development Plans 62-63 11. Community and other Stake holder participation 64-65 12. Linkages / Co-ordination with other agencies for Disaster Management 66 13. Budget and Other Financial allocation – Outlays of major schemes 67 14. Monitoring and Evaluation 68 15. Risk Communication Strategies 69-70 16. Important Contact Numbers and provision for link to detailed information 71-108 (All Line Department, BDO, EO, VAO’s) 17. Dos and Don’ts during all possible Hazards 109-115 18. Important Government Orders 116-117 19. Linkages with Indian Disaster Resource Network 118 20 Vulnerable Groups details 118 21. Mock Drill Schedules 119 22. Date of approval of DDMP by DDMA 120 23. Annexure 1 – 14 120-148 Tiruchirappalli District Disaster Management Plan – 2020 LIST OF ABBREVIATIONS S. Abbreviation Explanation No. 1. AO Agriculture Officer 2 AF Armed Forces 3 BDO Block Development Officers 4 DDMA District Disaster Management Authority 5 DDMP District Disaster Management Plan 6 DEOC District Emergency Operations Center 7 DRR Disaster Risk Reduction 8 DERAC District Emergency Relief Advisory Committee.