Genetic Disorders of Pigmentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D
Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D. Director of Ethnic Skin Care Voluntary Assistant Professor Miller/University of Miami School of Medicine Department of Dermatology and Cutaneous Surgery What Determines Skin Color? What Determines Skin Color? No significant difference in the number of melanocytes between the races 2000 epidermal melanocytes/mm2 on head and forearm 1000 epidermal melanocytes/mm2 on the rest of the body differences present at birth Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff,K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192-220, New York, NY: McGraw Hill Melanosomes in Black and White Skin Black White Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Nature1969;222:1081-1082 Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192- 220, New York, NY: McGraw Hill Role of Melanin-Advantages Melanin absorbs and scatters energy from UV and visible light to protect epidermal cells from UV damage Disadvantages Inflammation or injury to the skin is almost immediately accompanied by alteration in pigmentation Hyperpigmentation Hypopigmentation Dyschromias Post-Inflammatory hyperpigmentation Acne Melasma Lichen Planus Pigmentosus Progressive Macular Hypomelanosis -

Genetic Testing for Non-Cancerous Inheritable Diseases
Genetic Testing for Non-Cancerous Inheritable Diseases Policy Number: Original Effective Date: MM.02.009 03/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/30/2016 Section: Medicine Place(s) of Service: Outpatient I. Description A genetic test is the analysis of human DNA, RNA, chromosomes, proteins, or certain metabolites in order to detect alterations related to an inheritable disorder. This can be accomplished by directly examining the DNA or RNA that makes up a gene (direct testing), looking at markers co-inherited with a disease-causing gene (linkage testing), assaying certain metabolites (biochemical testing), or examining the chromosomes (cytogenetic testing). Genetic tests are conducted for a number of purposes, including predicting disease risk, newborn screening, determining clinical management, identifying carriers, and establishing prenatal or clinical diagnoses or prognoses in individuals, families, or populations. Expanded Carrier Screening The American College of Medical Genetics (ACMG ) defines expanded panels as those that use next- generation sequencing to screen for mutations in many genes, as opposed to gene-by-gene screening (e.g., ethnic-specific screening or panethnic testing for cystic fibrosis). An ACMG position statement states that although commercial laboratories offer expanded carrier screening panels, there has been no professional guidance as to which disease genes and mutations to include. This policy does not address oncology-related genetic testing or pre-implantation genetic diagnosis (PGD). II. Criteria/Guidelines Note: For familial assessments, unless otherwise specified, family will be considered: first-, second- and third- degree relatives on the same side of the family. The maternal and paternal sides should be considered independently. -

Natural Skin‑Whitening Compounds for the Treatment of Melanogenesis (Review)
EXPERIMENTAL AND THERAPEUTIC MEDICINE 20: 173-185, 2020 Natural skin‑whitening compounds for the treatment of melanogenesis (Review) WENHUI QIAN1,2, WENYA LIU1, DONG ZHU2, YANLI CAO1, ANFU TANG1, GUANGMING GONG1 and HUA SU1 1Department of Pharmaceutics, Jinling Hospital, Nanjing University School of Medicine; 2School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, Jiangsu 210002, P.R. China Received June 14, 2019; Accepted March 17, 2020 DOI: 10.3892/etm.2020.8687 Abstract. Melanogenesis is the process for the production of skin-whitening agents, boosted by markets in Asian countries, melanin, which is the primary cause of human skin pigmenta- especially those in China, India and Japan, is increasing tion. Skin-whitening agents are commercially available for annually (1). Skin color is influenced by a number of intrinsic those who wish to have a lighter skin complexions. To date, factors, including skin types and genetic background, and although numerous natural compounds have been proposed extrinsic factors, including the degree of sunlight exposure to alleviate hyperpigmentation, insufficient attention has and environmental pollution (2-4). Skin color is determined by been focused on potential natural skin-whitening agents and the quantity of melanosomes and their extent of dispersion in their mechanism of action from the perspective of compound the skin (5). Under physiological conditions, pigmentation can classification. In the present article, the synthetic process of protect the skin against harmful UV injury. However, exces- melanogenesis and associated core signaling pathways are sive generation of melanin can result in extensive aesthetic summarized. An overview of the list of natural skin-lightening problems, including melasma, pigmentation of ephelides and agents, along with their compound classifications, is also post‑inflammatory hyperpigmentation (1,6). -

Uniform Faint Reticulate Pigment Network - a Dermoscopic Hallmark of Nevus Depigmentosus
Our Dermatology Online Letter to the Editor UUniformniform ffaintaint rreticulateeticulate ppigmentigment nnetworketwork - A ddermoscopicermoscopic hhallmarkallmark ooff nnevusevus ddepigmentosusepigmentosus Surit Malakar1, Samipa Samir Mukherjee2,3, Subrata Malakar3 11st Year Post graduate, Department of Dermatology, SUM Hospital Bhubaneshwar, India, 2Department of Dermatology, Cloud nine Hospital, Bangalore, India, 3Department of Dermatology, Rita Skin Foundation, Kolkata, India Corresponding author: Dr. Samipa Samir Mukherjee, E-mail: [email protected] Sir, ND is a form of cutaneous mosaicism with functionally defective melanocytes and abnormal melanosomes. Nevus depigmentosus (ND) is a localized Histopathologic examination shows normal to hypopigmentation which most of the time is congenital decreased number of melanocytes with S-100 stain and and not uncommonly a diagnostic challenge. ND lesions less reactivity with 3,4-dihydroxyphenylalanine reaction are sometimes difficult to differentiate from other and no melanin incontinence [2]. Electron microscopic hypopigmented lesions like vitiligo, ash leaf macules and findings show stubby dendrites of melanocytes nevus anemicus. Among these naevus depigmentosus containing autophagosomes with aggregates of poses maximum difficulty in differentiating from ash melanosomes. leaf macules because of clinical as well as histological similarities [1]. Although the evolution of newer diagnostic For ease of understanding the pigmentary network techniques like dermoscopy has obviated the -

EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies
Focusing on Personalised Medicine EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies Extended carrier screening is an important tool for prospective parents to help them determine their risk of having a child affected with a heritable disease. In many cases, parents aren’t aware they are carriers and have no family history due to the rarity of some diseases in the general population. What is covered by the screening? Genomics For Life offers a comprehensive Extended Carrier Screening test, providing prospective parents with the information they require when planning their pregnancy. Extended Carrier Screening has been shown to detect carriers who would not have been considered candidates for traditional risk- based screening. With a simple mouth swab collection, we are able to test for over 419 genes associated with inherited diseases, including Fragile X Syndrome, Cystic Fibrosis and Spinal Muscular Atrophy. The assay has been developed in conjunction with clinical molecular geneticists, and includes genes listed in the NIH Genetic Test Registry. For a list of genes and disorders covered, please see the reverse of this brochure. If your gene of interest is not covered on our Extended Carrier Screening panel, please contact our friendly team to assist you in finding a gene test panel that suits your needs. Why have Extended Carrier Screening? Extended Carrier Screening prior to pregnancy enables couples to learn about their reproductive risk and consider a complete range of reproductive options, including whether or not to become pregnant, whether to use advanced reproductive technologies, such as preimplantation genetic diagnosis, or to use donor gametes. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -

Dermatologic Manifestations of Hermansky-Pudlak Syndrome in Patients with and Without a 16–Base Pair Duplication in the HPS1 Gene
STUDY Dermatologic Manifestations of Hermansky-Pudlak Syndrome in Patients With and Without a 16–Base Pair Duplication in the HPS1 Gene Jorge Toro, MD; Maria Turner, MD; William A. Gahl, MD, PhD Background: Hermansky-Pudlak syndrome (HPS) con- without the duplication were non–Puerto Rican except sists of oculocutaneous albinism, a platelet storage pool de- 4 from central Puerto Rico. ficiency, and lysosomal accumulation of ceroid lipofuscin. Patients with HPS from northwest Puerto Rico are homozy- Results: Both patients homozygous for the 16-bp du- gous for a 16–base pair (bp) duplication in exon 15 of HPS1, plication and patients without the duplication dis- a gene on chromosome 10q23 known to cause the disorder. played skin color ranging from white to light brown. Pa- tients with the duplication, as well as those lacking the Objective: To determine the dermatologic findings of duplication, had hair color ranging from white to brown patients with HPS. and eye color ranging from blue to brown. New findings in both groups of patients with HPS were melanocytic Design: Survey of inpatients with HPS by physical ex- nevi with dysplastic features, acanthosis nigricans–like amination. lesions in the axilla and neck, and trichomegaly. Eighty percent of patients with the duplication exhibited fea- Setting: National Institutes of Health Clinical Center, tures of solar damage, including multiple freckles, stel- Bethesda, Md (a tertiary referral hospital). late lentigines, actinic keratoses, and, occasionally, basal cell or squamous cell carcinomas. Only 8% of patients Patients: Sixty-five patients aged 3 to 54 years were di- lacking the 16-bp duplication displayed these findings. -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -
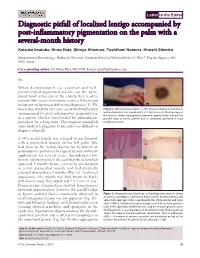
Diagnostic Pitfall of Localized Lentigo Accompanied by Post-Inflammatory
Letter to the Editor Diagnostic pitfall of localized lentigo accompanied by post-inflammatory pigmentation on the palm with a several-month history Keisuke Imafuku, Hiroo Hata, Shinya Kitamura, Toshifumi Nomura, Hiroshi Shimizu Department of Dermatology, Hokkaido University Graduate School of MedicineNorth 15, West 7, Kita-ku, Sapporo 060- 8638, Japan Corresponding author: Dr. Hiroo Hata, MD, PhD, E-mail: [email protected] Sir, When dermatologists see acquired and well- circumscribed pigmented macules on the palm, dorsal hand or forearm of the elderly, they tend to consider blue nevus, hematoma, nevus cell nevus and malignant melanoma as differential diagnoses [1]. We a b herein described the very rare case of localized lentigo Figure 1: Clinical manifestations. a. The macule is brown to dark black, accompanied by post-inflammatory pigmentation well-demarcated, flat, round and 3 x 4 mm in size. b. Dermoscopy of the macule shows homogenous brownish pigmentation without the in a patient who has been treated for palmoplanter parallel ridge or furrow pattern that is commonly observed in acral pustulosis for a long time. This eruption mimicked lentiginous lesion some kinds of lentiginous lesion and it was difficult to diagnose clinically. A 59-year-old female was referred to our hospital with a pigmented macule on her left palm. She had been to the family doctor for treatment of palmoplanter pustulosis by topical steroid ointment application for several years. According to the a history-taking interview, she said that the lesion had b appeared 5 months before referral to our hospital as a tiny pigmented macule and had gradually enlarged during those 5 months (Fig. -

Phacomatosis Spilorosea Versus Phacomatosis Melanorosea
Acta Dermatovenerologica 2021;30:27-30 Acta Dermatovenerol APA Alpina, Pannonica et Adriatica doi: 10.15570/actaapa.2021.6 Phacomatosis spilorosea versus phacomatosis melanorosea: a critical reappraisal of the worldwide literature with updated classification of phacomatosis pigmentovascularis Daniele Torchia1 ✉ 1Department of Dermatology, James Paget University Hospital, Gorleston-on-Sea, United Kingdom. Abstract Introduction: Phacomatosis pigmentovascularis is a term encompassing a group of disorders characterized by the coexistence of a segmental pigmented nevus of melanocytic origin and segmental capillary nevus. Over the past decades, confusion over the names and definitions of phacomatosis spilorosea, phacomatosis melanorosea, and their defining nevi, as well as of unclassifi- able phacomatosis pigmentovascularis cases, has led to several misplaced diagnoses in published cases. Methods: A systematic and critical review of the worldwide literature on phacomatosis spilorosea and phacomatosis melanorosea was carried out. Results: This study yielded 18 definite instances of phacomatosis spilorosea and 14 of phacomatosis melanorosea, with one and six previously unrecognized cases, respectively. Conclusions: Phacomatosis spilorosea predominantly involves the musculoskeletal system and can be complicated by neuro- logical manifestations. Phacomatosis melanorosea is sometimes associated with ancillary cutaneous lesions, displays a relevant association with vascular malformations of the brain, and in general appears to be a less severe syndrome. -

Iris Mammillations: Significance and Associations
IRIS MAMMILLATIONS: SIGNIFICANCE AND ASSOCIATIONS 2 l NICOLA K. RAGGEL2, 1. ACHESON and A. LINN MURPHREE Los Angeles and London SUMMARY mammiform (nipple- or teat-like) protuberances. Iris mammillations are rarely described, distinctive Iris mammillations are an occasional finding with few previous reports. They are most commonly villiform protuberances that can cover the iris. In the l--6 majority of reported cases they are unilateral and found in association with melanosis oculi, with or sporadic, and are seen in association with oculodermal without periocular skin involvement in a naevus of melanosis. In past literature and current clinical Ota. They are thus often less precisely referred to as practice they are frequently confused with tbe iris iris melanosis, a term which should best be reserved nodules seen in neurofibromatosis type 1. Their clinical for increased pigmentation of the iris, irrespective of significance is not established, although it has been the presence of iris elevations overlying the pigmen 7 suggested that iris mammillations may be an external ted areas. This is supported by the rare descriptions sign of ocular hypertension or intraocular malignancy. of iris elevations in the absence of any increased iris We report a series of 9 patients between the ages of 3 pigmentation?,8 and 28 years with iris mammillations. The mammilla Iris mammillations are usually unilateral, often tions appear as regularly spaced, deep brown, smooth, presenting as heterochromia iridis. Occasional bilat 7 conical elevations on the iris, of uniform height or eral cases have been described. ,8 Iris mammillations increasing in height as the pupil margin is approached.