Diffuse Esophageal Spasm and Detrusor Spasm: Combined Visceral Neuromuscular Disorders in a Patient with William’S Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laparoscopic Heller's Cardiomyotomy in Cirrhosis with Oesophageal Varices
Unusual Case Laparoscopic Heller’s cardiomyotomy in cirrhosis with oesophageal varices Abhay N Dalvi, Pinky M Thapar, Nitin M Narawane1, Rippan N Shukla Departments of Minimal Invasive Surgery and 1Gastroenterology, Jupiter Hospital, Thane, Maharashtra, India. Address for correspondence: Dr. Abhay N Dalvi, 257 Walkeshwar Road, Mumbai-400 006, India. E-mail: [email protected] Abstract in the presence of varices is technically challenging. Bleeding obscuring the vision is one of the obstacles Surgical intervention in cirrhosis of liver with of this procedure that can lead to complication portal hypertension is associated with increased morbidity and mortality. This is attributed to of oesophageal mucosal perforation. Thorough liver decompensation, intra-operative bleeding, pre-operative investigations, planning, meticulous prolonged operative time, wound related and dissection are required to tackle this problem by a anaesthesia complications. Laparoscopic surgery laparoscopic approach. PUBMED search shows no in cirrhosis is advantageous but is associated with reported case of laparoscopic cardiomyotomy in patient technical challenges. We report one such case of cirrhosis with oesophageal varices. of hepatitis C cirrhosis with oesophageal varices and symptomatic achalasia cardia, who was successfully treated by laparoscopic cardiomyotomy CASE REPORT after thorough preoperative workup and planning. In the review of literature on pubmed, no such case A 53-year-old lady was referred to us for surgical is reported.. management of achalasia cardia. In the past, she had sustained severe gastroenteritis (30 years ago) for which Key words: Achalasia, cirrhosis, esophageal varices, laparoscopic cardiomyotomy. she was transfused 2 units of blood. She developed jaundice 25 years ago which responded to medical DOI: 10.4103/0972-9941.65164 management. -

The Gastrointestinal System and the Elderly
2 The Gastrointestinal System and the Elderly Thomas W. Sheehy 2.1. Introduction Gastrointestinal diseases increase with age, and their clinical presenta tions are often confused by functional complaints and by pathophysio logic changes affecting the individual organs and the nervous system of the gastrointestinal tract. Hence, the statement that diseases of the aged are characterized by chronicity, duplicity, and multiplicity is most appro priate in regard to the gastrointestinal tract. Functional bowel distress represents the most common gastrointestinal disorder in the elderly. Indeed, over one-half of all their gastrointestinal complaints are of a functional nature. In view of the many stressful situations confronting elderly patients, such as loss of loved ones, the fears of helplessness, insolvency, ill health, and retirement, it is a marvel that more do not have functional complaints, become depressed, or overcompensate with alcohol. These, of course, make the diagnosis of organic complaints all the more difficult in the geriatric patient. In this chapter, we shall deal primarily with organic diseases afflicting the gastrointestinal tract of the elderly. To do otherwise would require the creation of a sizable textbook. THOMAS W. SHEEHY • Birmingham Veterans Administration Medical Center; and University of Alabama in Birmingham, School of Medicine, Birmingham, Alabama 35233. 63 S. R. Gambert (ed.), Contemporary Geriatric Medicine © Plenum Publishing Corporation 1988 64 THOMAS W. SHEEHY 2.1.1. Pathophysiologic Changes Age leads to general and specific changes in all the organs of the gastrointestinal tract'! Invariably, the teeth show evidence of wear, dis cloration, plaque, and caries. After age 70 years the majority of the elderly are edentulous, and this may lead to nutritional problems. -

Practical Approaches to Dysphagia Caused by Esophageal Motor Disorders Amindra S
Practical Approaches to Dysphagia Caused by Esophageal Motor Disorders Amindra S. Arora, MB BChir and Jeffrey L. Conklin, MD Address nonspecific esophageal motor disorders (NSMD), diffuse Division of Gastroenterology and Hepatology, Mayo Clinic, esophageal spasm (DES), nutcracker esophagus (NE), 200 First Street SW, Rochester, MN 55905, USA. hypertensive lower esophageal sphincter (hypertensive E-mail: [email protected] LES), and achalasia [1••,3,4••,5•,6]. Out of all of these Current Gastroenterology Reports 2001, 3:191–199 conditions, only achalasia can be recognized by endoscopy Current Science Inc. ISSN 1522-8037 Copyright © 2001 by Current Science Inc. or radiology. In addition, only achalasia has been shown to have an underlying distinct pathologic basis. Recent data suggest that disorders of esophageal motor Dysphagia is a common symptom with which patients function (including LES incompetence) affect nearly present. This review focuses primarily on the esophageal 20% of people aged 60 years or over [7••]. However, the motor disorders that result in dysphagia. Following a brief most clearly defined motility disorder to date is achalasia. description of the normal swallowing mechanisms and the Several studies reinforce the fact that achalasia is a rare messengers involved, more specific motor abnormalities condition [8•,9]. However, no population-based studies are discussed. The importance of achalasia, as the only exist concerning the prevalence of most esophageal motor pathophysiologically defined esophageal motor disorder, disorders, and most estimates are derived from people with is discussed in some detail, including recent developments symptoms of chest pain and dysphagia. A recent review of in pathogenesis and treatment options. Other esophageal the epidemiologic studies of achalasia suggests that the spastic disorders are described, with relevant manometric worldwide incidence of this condition is between 0.03 and tracings included. -

Adverse Events of Upper GI Endoscopy
GUIDELINE Adverse events of upper GI endoscopy This is one of a series of statements discussing the use of lications rely on self-reporting, and most reported data GI endoscopy in common clinical situations. The Stan- collected only from the immediate periprocedure period, dards of Practice Committee of the American Society for thus the rate of late adverse events and mortality may be Gastrointestinal Endoscopy (ASGE) prepared this text. underestimated.8,9 Major adverse events related to diag- In preparing this document, a search of the medical liter- nostic UGI endoscopy are rare and include cardiopulmo- ature was performed by using PubMed. Additional refer- nary adverse events, infection, perforation, and bleeding. ences were obtained from the bibliographies of the identi- Adverse events of ERCP and EUS are discussed in separate fied articles and from recommendations of expert ASGE documents.10,11 consultants. When few or no data exist from well-designed prospective trials, emphasis is given to results of large series and reports from recognized experts. This document is ADVERSE EVENTS ASSOCIATED WITH based on a critical review of the available data and expert DIAGNOSTIC UGI ENDOSCOPY consensus at the time that the document was drafted. Further controlled clinical studies may be needed to clar- Cardiopulmonary adverse events ify aspects of this document. This document may be re- Most UGI procedures in the United States and Europe vised as necessary to account for changes in technology, are performed with patients under sedation (moderate or 12 new data, or other aspects of clinical practice. deep). Cardiopulmonary adverse events related to seda- This document is intended to be an educational device tion and analgesia account for as much as 60% of UGI 1-4,7 to provide information that may assist endoscopists in endoscopy adverse events. -

The Gastrointestinal Tract Frank A
91731_ch13 12/8/06 8:55 PM Page 549 13 The Gastrointestinal Tract Frank A. Mitros Emanuel Rubin THE ESOPHAGUS Bezoars Anatomy THE SMALL INTESTINE Congenital Disorders Anatomy Tracheoesophageal Fistula Congenital Disorders Rings and Webs Atresia and Stenosis Esophageal Diverticula Duplications (Enteric Cysts) Motor Disorders Meckel Diverticulum Achalasia Malrotation Scleroderma Meconium Ileus Hiatal Hernia Infections of the Small Intestine Esophagitis Bacterial Diarrhea Reflux Esophagitis Viral Gastroenteritis Barrett Esophagus Intestinal Tuberculosis Eosinophilic Esophagitis Intestinal Fungi Infective Esophagitis Parasites Chemical Esophagitis Vascular Diseases of the Small Intestine Esophagitis of Systemic Illness Acute Intestinal Ischemia Iatrogenic Cancer of Esophagitis Chronic Intestinal Ischemia Esophageal Varices Malabsorption Lacerations and Perforations Luminal-Phase Malabsorption Neoplasms of the Esophagus Intestinal-Phase Malabsorption Benign tumors Laboratory Evaluation Carcinoma Lactase Deficiency Adenocarcinoma Celiac Disease THE STOMACH Whipple Disease Anatomy AbetalipoproteinemiaHypogammaglobulinemia Congenital Disorders Congenital Lymphangiectasia Pyloric Stenosis Tropical Sprue Diaphragmatic Hernia Radiation Enteritis Rare Abnormalities Mechanical Obstruction Gastritis Neoplasms Acute Hemorrhagic Gastritis Benign Tumors Chronic Gastritis Malignant Tumors MénétrierDisease Pneumatosis Cystoides Intestinalis Peptic Ulcer Disease THE LARGE INTESTINE Benign Neoplasms Anatomy Stromal Tumors Congenital Disorders Epithelial Polyps -

Chest Pain of Gastrointestinal Origin
Arch Dis Child: first published as 10.1136/adc.63.12.1457 on 1 December 1988. Downloaded from Archives of Disease in Childhood, 1988, 63, 1457-1460 Chest pain of gastrointestinal origin S BEREZIN, M S MEDOW, M S GLASSMAN, AND L J NEWMAN Department of Pediatrics, New York Medical College, Valhalla, New York, USA SUMMARY Twenty seven children who had been diagnosed as having idiopathic chest pain were investigated to find out if the pain was of gastrointestinal origin. The symptoms had lasted from two weeks to eight months. In 21 of the 27 children (78%) the chest pain had a gastrointestinal cause: 16 had oesophagitis, four had gastritis, and one had diffuse oesophageal spasm. All patients responded to medical treatment of their gastrointestinal symptoms, resulting in disappearance of the chest pain. Although chest pain is not usually associated with the central or retrosternal areas, or both, and serious disease in children, if it occurs it is a cause of episodes of pain lasted from less than a minute to great anxiety for the patient and family. In one study several hours (table 1). 16% of children with chest pain made more than one In order to determine the cause of the pain, each visit to the accident and emergency department.' patient was investigated by oesophagogastroduode- Gastrointestinal disorders have not been de- noscopy, oesophageal manometry, and a Bernstein copyright. scribed as being important causes of chest pain in acid perfusion test. In addition, eight patients had children.1-3 To further our understanding of the extended ambulatory pH monitoring. -

The First-Bite Syndrome
Henry Ford Hospital Medical Journal Volume 34 Number 4 Article 12 12-1986 The First-Bite Syndrome William S. Haubrich Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Haubrich, William S. (1986) "The First-Bite Syndrome," Henry Ford Hospital Medical Journal : Vol. 34 : No. 4 , 275-278. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol34/iss4/12 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. The First-Bite Syndrome William S. Haubrich, MD^ Patients presenting with esophageal disorders often describe what can be called a "first-bite syndrome." The condition can be discerned by its characteristic clinical features. It may be a variant of diffuse esophageal spasm. While in a majority of patients it is a benign functional disturbance, it can be a harbinger of carcinoma. When of functional origin, it is amenable, in most cases, to relatively simple medical management. (Henry Ford Hosp MedJ 1986;34:275-8) syndrome is a concunence, in Greek literally "a mnning The survey included a review of symptoms, physical find A: Ltogether," of symptoms or signs that in a given patient ings, laboratory data, and the findings at radiography or endos come to indicate a particular abnormality. The more often one copy of the proximal alimentary tract. elicits from a series of patients a consistent set of symptoms, the The clinical criteria for identifying patients who exhibited the more convinced one becomes that the concunence is significant FBS were; 1) repeated episodes of dysphagia with retrostemal and not merely coincidence. -

Esophagus Stomach/Small Bowel Colon
10/29/2012 Barry Schlansky, M.D. Oregon Health and Science University Esophagus . Dysphagia . GERD / Barrett’s esophagus Stomach/Small Bowel . Peptic Ulcer Disease . Gastric cancer . GI Bleeding . Celiac Disease Colon . Colorectal Cancer Screening . Irritable Bowel Disease . Inflammatory Bowel Disease . Infectious Diarrhea . Diverticular Disease . Anorectal Disease 1 10/29/2012 Dysphagia Esophageal Oropharyngeal Solids only Liquids and Solids •CVA Progressive Intermittent Progressive Intermittent •Neuromuscular Disorder •ENT Cancer • Peptic • Schatzki Ring • Achalasia • Diffuse •Speech Eval Stricture •Scleroderma Esophageal •Modified Spasm Barium Swallow •Esophageal •GERD-related Tumor dysmotility •Nonspecific Dysmotility Aperistaltic esophagus with impaired relaxation fo the lower esophageal sphincter (LES) Diagnosis . UGI Barium – “bird’s beak” . EGD can suggest . Esophageal manometry Treatment . Nitrates, Calcium channel blockers . Botox injection of LES . Pneumatic dilation . Heller myotomy (cut LES) Differentiated using esophageal manometry Diffuse Esophageal Spasm: chest pain > dysphagia . High-amplitude simultaneous peristaltic waves . Corkscrew appearance on UGI Nutcracker esophagus: . Chest pain > dysphagia . Normal peristalsis but high-amplitude and prolonged duration of peristaltic waves 2 10/29/2012 Epidemiology . M=F . All ages equally Risk Factors . Obesity . Smoking . Alcohol use . Pregnancy . Foods: spicy, tomatoes, peppermint, caffeine, fat, chocolate . Recumbency . Delayed gastric emptying (narcotics, DM) -
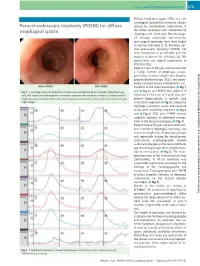
For Diffuse Esophageal Spasm… Endoscopy 2014; 46: E79–E81 E80 Cases and Techniques Library (CTL)
Cases and Techniques Library (CTL) E79 Diffuse esophageal spasm (DES) is a rare esophageal dysmotility condition charac- Peroral endoscopic myotomy (POEM) for diffuse terized by simultaneous contractions of esophageal spasm the distal esophagus and manifested by dysphagia and chest pain. Pharmacologi- cal therapy, endoscopic interventions, and surgical myotomy have been linked to various outcomes [1,2]. Recently, per- oral endoscopic myotomy (POEM) has been introduced as an effective and less invasive treatment for achalasia [3]. We report here our clinical experiences of POEM for DES. Patient 1 was an 84-year-old woman with a 5-year history of dysphagia accom- panied by excessive weight loss. Esopha- gogastroduodenoscopy (EGD) and mano- metry revealed severe simultaneous con- tractions in the lower esophagus (●" Fig.1 ●" Fig. 1 Left image shows the abnormal simultaneous contractions before peroral endoscopic myot- and Fig.2) and POEM was applied. A omy. The scope passed through the contraction segment with moderate resistance. Post-procedural myotomy of 15.0 cm in length was per- esophagoscopy showed that the contractions were not seen on the anterior side of the muscle incision formed longitudinally to include each (right image). contraction segment (●" Fig.3). Subjective dysphagia symptom scores and pressure study were markedly improved (●" Fig.2 and ●" Fig.4). EGD after POEM showed complete absence of abnormal contrac- tions in the incised esophagus (●" Fig.1). Patient 2 was a 79-year-old man with a 20- year history of dysphagia, vomiting, and excessive weight loss. Endoscopic passage was impossible during the simultaneous contractions. Esophagography showed corkscrew-shaped contractions and block- age of barium passage at the proximal por- tion of contractions (●" Fig. -

Esophageal Manometry Among Patients with Dysphagia Referred to Kurdistan Center for Gastroenterology and Hepatology
Esophageal Manometry Among Patients With Dysphagia Referred To Kurdistan Center For Gastroenterology And Hepatology Mohammed O. Mohammed*, Bakhtyar F. Salim*, Ali A. Ramadhan ** Abstract Background and Objectives Dysphagia is a common problem in patients with primary motor disorders of the esophagus. Esophageal manom- etry is the gold standard for diagnosis of these disorders. Introduction of high resolution manometry represented a significant improvement in data recording and diagnostic yield. The objective of the study was to assess the findings of esophageal high resolution impedance manometry in patients presenting with dysphagia in Sulaimani governorate. Patients and Methods This study extended from September, 2012 to December, 2013 and included 120 patients with dysphagia who were referred for manometry in Kurdistan Center for Gastroenterology and Hepatology (KCGH) in Sulaimani city. All patients underwent upper endoscopy to exclude mechanical and inflammatory causes of dysphagia then the high resolution impedance manometry was used with liquid and viscous swallows. Results The mean age of the study population was 43 years. The female to male ratio was 1.5:1. The mean duration of dysphagia was 2 years. The most common esophageal motility abnormality was achalasia (N=44, 36.7%) followed by hypertensive LES (N=31, 25.8%), ineffective esophageal motility (N=9, 7.5%), hypotensive LES (N=5, 4.2%) and diffuse esophageal spasm (N=3, 2.5%). The high resolution impedance manometry was normal in 28 patients (23.3%). Of the 44 patients with achalasia, 15 patients (34%) had vigorous achalasia. Using Chica- go classification, the most common type of achalasia was type II (N=26, 59%) followed by type I (N=13, 29.6%) and then type III (N=5, 11.4%). -

Progression of Jackhammer Esophagus to Type II Achalasia
J Neurogastroenterol Motil, Vol. 22 No. 1 January, 2016 pISSN: 2093-0879 eISSN: 2093-0887 http://dx.doi.org/10.5056/jnm15162 JNM Journal of Neurogastroenterology and Motility Case Report Progression of Jackhammer Esophagus to Type II Achalasia Jason Abdallah and Ronnie Fass* The Esophageal and Swallowing Center, Division of Gastroenterology and Hepatology, MetroHealth Medical Center, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA It has been suggested that patients with certain motility disorders may progress overtime to develop achalasia. We describe a 66 year-old woman who presented with dysphagia for solids and liquids for a period of 18 months. Her initial workup showed normal endoscopy and non-specific esophageal motility disorder on conventional manometry. Six months later, due to persistence of symptoms, the patient underwent a high resolution esophageal manometry (HREM) demonstrating jackhammer esophagus. The patient was treated with a high dose proton pump inhibitor but without resolution of her symptoms. During the last year, the patient reported repeated episodes of food regurgitation and a significant weight loss. A repeat HREM revealed type II achalasia. Multiple case reports, and only a few prospective studies have demonstrated progression from certain esophageal motility disorders to achalasia. However, this report is the first to describe a case of jackhammer esophagus progressing to type II achalasia. (J Neurogastroenterol Motil 2016;22:153-156) Key Words Esophageal achalasia; Esophageal motility disorders; Esophagus; Manometry reflux disease (GERD) progressing to achalasia.4-9 Although no causal relationship has been identified, these reports suggest that the Introduction different esophageal motor disorders represent a spectrum rather Achalasia is a primary esophageal motor disorder of unknown than unique and stable disorders. -

Case Studies and Review of Jackhammer Esophagus
Review articles Case Studies and Review of Jackhammer Esophagus Robin Germán Prieto Ortiz, MD1, Álvaro Andrés Gómez Venegas, MD2, Albis Cecilia Hani de Ardila, MD3 1 General Surgeon and Resident in Gastroenterology at Abstract the Fundación Universitaria Sanitas 2 Internist and Gastroenterologist at Gastroclínico Jackhammer esophagus is a peristaltic hypercontractile disorder. According to the second version of the Chicago Institute in Medellín, Colombia Classification of esophageal motility, jackhammer esophagus is defined manometrically by distal contractile in- 3 Internist and Gastroenterologist, Director of the Unit tegrals greater than 8000 mm Hg/cm/s which indicates very high amplitude and velocity. We present a series of of Gastroenterology and Digestive Physiology of the Hospital Universitario San Ignacio in Bogotá, five patients with jackhammer esophagus who underwent high-resolution esophageal manometry (HREM) from Colombia which clinical and manometric data were collected. There were three men and two women whose ages ranged from 41 to 73. Three of them had been diagnosed with gastroesophageal reflux disease, and showed symptoms This work was presented as a poster at the 2015 ACADI Convention. of dysphagia, heartburn and regurgitation. The main endoscopic finding was the presence of hiatal hernia and presbyesophagus in two patients. HREM showed waves of up to 4 mm Hg greater than 8000 mm Hg/cm/s. In three of the five patients there were multiple waves. Although, the new third version of the Chicago classification ......................................... Received: 24-09-15 of requires two waves with DCIs over 8000 mm Hg/cm/s to confirm a diagnosis of jackhammer esophagus, it Accepted: 25-07-16 should be noted that we do not yet have available equipment to interpret MAR and allow classifying esophageal disorders by Chicago v.3, and that is why in our physiology unit we still report the MAR with presorting.