Predation of Artificial Xantus's Murrelet ( Synthliboramphus Hypoleucus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Cyanoramphus Novaezelandiae) from Motuihe Island to Little Barrier Island, New Zealand
48 Notornis, 2010, Vol. 57: 48-49 0029-4470 © The Ornithological Society of New Zealand, Inc. SHORT NOTE Homing of a red-crowned parakeet (Cyanoramphus novaezelandiae) from Motuihe Island to Little Barrier Island, New Zealand LUIS ORTIZ-CATEDRAL Ecology and Conservation Group, Institute of Natural Sciences, Massey University, Private Bag 102-904 North Shore Mail Centre, Auckland, New Zealand The red-crowned parakeet (Cyanoramphus parakeets were released by members of the novaezelandiae) is New Zealand’s most widespread Motuihe Island Trust and the general public in parakeet species, with a range extending from the a remnant of coastal bush on the west side of the Kermadecs Archipelago, across the North and island. In Apr 2009, I returned to Little Barrier I South Is, to the Chatham and Antipodes Is (Higgins with a team of volunteers to capture parakeets 1999; Juniper & Parr 1998). As the species has destined for translocation to Tawharanui Regional declined on the main islands of New Zealand, it has Park. Mist netting took place between 21 and 25 been translocated to a number of offshore islands Apr in Te Maraeroa flats. On 23 Apr, a banded over the last 40 years including Cuvier, Matiu/ female was captured and confirmed as one of Somes, Tiritiri Matangi and Whale Is (Dawe 1979; the parakeets released on Motuihe I the previous McHalick 1999; Miskelly et al. 2005). In May 2008, month. Thus, this bird had flown a minimum of a group of 31 red-crowned parakeets captured on ca. 65 km between Motuihe and Little Barrier Is. Little Barrier I was released on Motuihe I as part of On recapture on Little Barrier I, the recaptured an island restoration project. -

Oceania Species ID Sheets
Species Identification Sheets for Protected Wildlife in Trade - Oceania - 3 Mark O’Shea 1 Mike McCoy © Phil Bender 5 Tony Whitaker © 2 4 Tony Whitaker © 6 WILDLIFE ENFORCEMENT GROUP (AGRICULTURE & FORESTRY · CONSERVATION · N. Z. CUSTOMS SERVICE) Numbered images above Crown Copyright: Department of Conservation Te Papa Atawhai. Photographers:1) Dick Veitch 1981, 2) Rod Morris 1984, 3) Gareth Rapley 2009, 4) Andrew Townsend 2000, 5) Paul Schilov 2001, 6) Dick Veitch 1979 Introduction Purpose of this resource: - Additional species that should be included in this booklet Wildlife trafficking is a large-scale multi-billion dollar industry worldwide. The illegal trade of - Sources of information, such as identification guides or reports, related to these wildlife has reached such prominence that it has the potential to devastate source populations species of wildlife, impacting on the integrity and productivity of ecosystems in providing food and - Domestic legislation regarding the regulation of trade in wildlife - Sources of photographs for identification purposes resources to the local economy. In order to protect these resources, legislation has been put in place to control the trade of wildlife in almost every country worldwide. Those assigned with - Details of wildlife seizures, including the smuggling methods enforcing these laws have the monumental task of identifying the exact species that are being traded, either as whole living plants or animals, as parts that are dried, fried or preserved, or as Any feedback can be provided directly to the Wildlife Enforcement Group: derivatives contained within commercial products. Stuart Williamson Senior Investigator, Wildlife Enforcement Group This booklet “Species Identification Sheets for Protected Species in Trade – Oceania” has been Customhouse, Level 6, 50 Anzac Avenue, Auckland, New Zealand developed to address the lack of resources, identified by customs agencies within Oceania, for Ph: +64 9 3596676, Fax: +64 9 3772534 identification of wildlife species in trade. -

Exponential Population Increase in the Endangered Ouvéa Parakeet (Eunymphicus Uvaeensis) After Community-Based Protection From
J Ornithol DOI 10.1007/s10336-010-0499-7 ORIGINAL ARTICLE Exponential population increase in the endangered Ouve´a Parakeet (Eunymphicus uvaeensis) after community-based protection from nest poaching Nicolas Barre´ • Jo¨rn Theuerkauf • Ludovic Verfaille • Pierre Primot • Maurice Saoumoe´ Received: 20 July 2009 / Revised: 16 January 2010 / Accepted: 1 February 2010 Ó Dt. Ornithologen-Gesellschaft e.V. 2010 Abstract The Ouve´a Parakeet (Eunymphicus uvaeensis), Introduction endemic to Ouve´a Island (Loyalty Islands, New Caledonia, south-west Pacific), is a rainforest bird that is dependent on The Ouve´a Parakeet (Eunymphicus uvaeensis) is endemic tree cavities for nesting. It is threatened by deforestation, to the small island of Ouve´a (Loyalty Islands, New Cale- but also by competition for nest sites with introduced bees, donia, south-west Pacific). The species was previously harvesting for pets, and potentially predation by introduced considered a subspecies of the Horned Parakeet (Eunym- species. Despite these threats, we show that the Ouve´a phicus cornutus), which is endemic to mainland New Parakeet population increased exponentially from an esti- Caledonia (Grande Terre). The Ouve´a Parakeet is now mated 617 (274–996) birds in 1993 to 2,090 (1,280–3,413) recognized as a full species (Juniper and Parr 1998; Boon birds in 2009 (95% confidence interval). We explain this et al. 2008). The two species are the only taxa of the genus population increase by community-based protection mea- Eunymphicus. sures that eliminated nest poaching. We recommend that The Ouve´a Parakeet is a rainforest species that nests in these measures are maintained, remnant forest is protected, cavities in mature trees (Robinet et al. -

Biosecurity Plan for Ouvea Atoll, Loyalty Islands, New Caledonia
Biosecurity Plan for Ouvea Atoll, Loyalty Islands, New Caledonia Souad Boudjelas and Karyn Froud May 2016 Prepared in partnership with the Pacific Community with support from the European Union under the INTEGRE Project Citation: Boudjelas1, S. and Froud2, K. (2016). Biosecurity Plan for Ouvea Atoll, Loyalty islands, New Caledonia. pp.68. Prepared for the Pacific Community. Auckland, New Zealand. 1 Pacific Invasives Initiative (PII), Auckland, New Zealand. 2 Biosecurity Research Ltd., Auckland, New Zealand. Reviewers: - Yolaine Bouteiller, Pacific Community, Noumea, New Caledonia. - Luen Iopué, Province des Iles Loyauté, Lifou, New Caledonia. - Antoine Barnaud, Province des Iles Loyauté, Lifou, New Caledonia. - Christine Fort, Conservatoire d’Espaces Naturels, Koné, New Caledonia - Aurelie Chan, Service d'Inspection Vétérinaire, Alimentaire et Phytosanitaire, Noumea, New Caledonia. Version History: Revisions to the Biosecurity Plan should be saved as new versions and recorded in the following table: VERSION DATE AUTHOR COMMENTS Ouvea_Biosecurity_Plan_181215 18/12/15 Souad Boudjelas Prepared following and Karyn completion of a report on Froud findings from a literature review, stakeholder consultation (see APPENDIX 2) and visits to facilities in Lifou, Ouvea and Noumea between 19th and 24th July 2015. Ouvea_Biosecurity_Plan_100516 10/05/2016 Souad Boudjelas Revised based on feedback and Karyn from stakeholder consultation Froud (see APPENDIX 3) in Ouvea and Noumea between 22nd and 26th February 2016 and reviewers listed above. TABLE OF -

New Caledonia, Fiji, Vanuatu & Samoa
The incomparable Kagu (Mark Van Beirs) NEW CALEDONIA, FIJI, VANUATU & SAMOA 7 – 30 AUGUST 2014 LEADER: MARK VAN BEIRS Our New Caledonia, Fiji, Vanuatu and Samoa tour offers the best of South Pacific birding with well-behaved flightless, brilliantly-coloured or mega skulking species, comfortable lodging, smooth travelling, glorious scenery, lush lowland and dripping montane forests, turquoise seas, palm-lined white beaches, impressive shield volcanoes and a peculiar mix of Melanesian and Polynesian culture. On our recent tour we visited ten quite different islands in four diverse countries, took dozens of flights and observed most of the extant endemic birds. The Vanuatu lowlands produced the shy Vanuatu Megapode, Tanna Fruit Dove, the cracking Vanuatu Kingfisher and the adorable Buff-bellied Monarch, and an exploration of the middle altitudes gave us Vanuatu Imperial Pigeon. On the mainland of New Caledonia the unique Kagu stole the show. This relic from the days when flightless birds ruled the world performed incredibly well. Other megas included White- bellied Goshawk, the incredibly attractive Cloven-feathered Dove and the very rare Crow Honeyeater. Our 1 BirdQuest Tour Report: New Caledonia, Fiji, Vanuatu & Samoa www.birdquest-tours.com short visits to the Loyalty Islands yielded Gould’s and Tahiti Petrels, the unique Ouvea Parakeet and the rarely-seen Large Lifou White-eye. Fiji’s Viti Levu island gave us lovely Golden Fruit Dove, Sulphur-breasted Myzomela, the very vocal Giant Honeyeater, the scarce Black-throated Shrikebill, Azure-crested Flycatcher and the retiring Long-legged Thicketbird. On Taveuni, the Garden Isle, the unbelievable Orange Fruit Dove impressed us most next to the avian wonder of the magnificent Silktail. -

The Ouvéa Parakeet
ORYX VOL 29 NO 2 APRIL 1995 The Ouvea parakeet - state of knowledge and conservation status O. Robinet, F. Beugnet, D. Dulieu and Ph. Chardonnet New Caledonia, a French territory in the south-west Pacific has a very high number of endemic taxa. The endemic fauna include a monotypic genus of parakeets - Eunymphicus. One subspecies, Eunymphicus cornutus uvaeensis, which is endemic to the island of Ouvea in the Loyalty Islands, is seriously threatened by degradation of its natural habitat, natural predators and capture for sale to collectors. There are now only 200-500 individuals left in the wild. The parakeet is the emblem of Ouvea and local people, together with research scientists, have formed a society with the aims of studying the parakeet in its natural environment, making the general public aware of its conservation requirements, combating smuggling, increasing its population by breeding it in captivity and, if possible, introducing it on to a neighbouring island. Introduction up of two parts linked by a narrow strip of land. This island, with 27 Melanesian inhabi- The Ouvea parakeet Eunymphicus cornutus tants per sq km, has a population density uvaeensis and the horned parakeet E. c. cornu- three times that of the rest of the Loyalty tus are the only two members of an endemic Islands (CTRDP, 1987; Mathieu-Daude, 1989). New Caledonian genus. The genus is related The parakeet's distribution is restricted to to Platycercus of Australia and Cyanoramphus Ouvea. It appears to have never colonized of New Zealand by its form and colouring, neighbouring islands despite their proximity which is mostly green with a red forehead and (45 km). -

New Caledonia & Fiji REP 16
The undoubted star of this fantastic tour is the unique KAGU (János Oláh). NEW CALEDONIA, FIJI VANUATU & SAMOA 4 / 7 – 20 / 23 AUGUST 2016 LEADER: JÁNOS OLÁH This itinerary targets a selection of special birds in a hidden corner of the world! It is is a superb tour to the South Pacific where, with the help of island-hopping, we encounter a new avifauna from time to time so the excitement never ends – until the very end of the tour anyway! For a split second I was wondering which stunning species to put on the front of the report but very quickly realised it can only be the unique Kagu no matter how many other goodies we saw on this birding tour. This is such a charismatic and amazing bird and the undoubted highlight for most birders on this tour! Nevertheless there is a fine selection of pigeons, doves, whistlers, shrikebills, honeyeaters and parrotfinches on this action-packed tour. Of course island hopping on this large scale (we visited nine different islands on this tour) can cause problems as well, and this year we were unlucky with our flights. While the start of the tour was ultimately not hampered by a cancelled flight, the last destination was affected for most of us. This fantastic region has many interesting birds with comfortable lodging, glorious scenery and a peculiar mix of Melanesian and Polynesian culture. We managed to see most of our targets, doing particularly well on Vanuatu, New Caledonia and Fiji where we cleaned up on all gettable specialties and only two special birds were missed on Samoa – which is a great result for a leaderless party! The total trip list was 141 recorded species with only one ’heard only’. -

Transfer of Eunymphicus Cornutus Uveaensis from Appendix II to Appendix I
Prop. 11.34 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II Other proposals A. Proposal Transfer of Eunymphicus cornutus uveaensis from Appendix II to Appendix I. The Uvéa parakeet has been and will continue to be affected by trade. The wild population is very small and is vulnerable because it is found only at a few places and its habitat is very reduced. B. Proponent France, at the request of New Caledonia C Supporting Statement 1. Taxonomy 1.1. Class: Aves 1.2. Order: Psittaciformes 1.3. Family: Psittacidaes 1.4. Genus: Eunymphicus species: Eunymphicus cornutus subspecies: Eunymphicus cornutus uveaensis Layard and Layard1882 1.5 Scientific synonyms: 1.6 Common names: English: Uvéa horned parakeet, Uvéa parakeet French: Perruche d'Ouvéa, Nymphique d'Ouvéa Spanish: Perico cornido de Uvéa German: Uvea-Hornsittich 1.7 Code numbers: CITES A-218.003.022.001 2. Biological Parameters 2.1 Distribution The Uvéa parakeet is endemic to the island of Uvéa in the Loyalty Islands. Its main population is found in the "Grande forêt" in the northern part of the island, an area of about 2000 hectares (Robinet et al. 1996), but some parakeets (including breeding couples) are also found in strips of forest on the isthmus and even in the southern part of the island. 2.2 Habitat availability The typical habitat of this parakeet is the primary forest and forest in all stages of degradation, from fallow Melanesian fields to secondary forest. This species lives primarily under the forest canopy. Its distribution is very heterogenous. This type of habitat covers 2000 hectares in the northern part of the island and 4600 hectare in the southern part, for a total of only 6600 hectares of potential habitat (Robinet et al. -

Exponential Population Increase in the Endangered Ouvéa
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by HAL-CIRAD Exponential population increase in the endangered Ouv´eaParakeet () after community-based protection from nest poaching Nicolas Barr´e,J¨ornTheuerkauf, Ludovic Verfaille, Pierre Primot, Maurice Saoumo´e To cite this version: Nicolas Barr´e,J¨ornTheuerkauf, Ludovic Verfaille, Pierre Primot, Maurice Saoumo´e.Exponen- tial population increase in the endangered Ouv´eaParakeet () after community-based protection from nest poaching. Journal f¨urOrnithologie = Journal of Ornithology, Springer Verlag, 2010, 151 (3), pp.695-701. <10.1007/s10336-010-0499-7>. <hal-00574835> HAL Id: hal-00574835 https://hal.archives-ouvertes.fr/hal-00574835 Submitted on 9 Mar 2011 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. J Ornithol (2010) 151:695–701 DOI 10.1007/s10336-010-0499-7 ORIGINAL ARTICLE Exponential population increase in the endangered Ouve´a Parakeet (Eunymphicus uvaeensis) after community-based protection from nest poaching Nicolas Barre´ • Jo¨rn Theuerkauf • Ludovic Verfaille • Pierre Primot • Maurice Saoumoe´ Received: 20 July 2009 / Revised: 16 January 2010 / Accepted: 1 February 2010 / Published online: 9 March 2010 Ó Dt. -

The Ouvéa Parakeet
ORYX VOL 29 NO 2 APRIL 1995 The Ouvea parakeet - state of knowledge and conservation status O. Robinet, F. Beugnet, D. Dulieu and Ph. Chardonnet New Caledonia, a French territory in the south-west Pacific has a very high number of endemic taxa. The endemic fauna include a monotypic genus of parakeets - Eunymphicus. One subspecies, Eunymphicus cornutus uvaeensis, which is endemic to the island of Ouvea in the Loyalty Islands, is seriously threatened by degradation of its natural habitat, natural predators and capture for sale to collectors. There are now only 200-500 individuals left in the wild. The parakeet is the emblem of Ouvea and local people, together with research scientists, have formed a society with the aims of studying the parakeet in its natural environment, making the general public aware of its conservation requirements, combating smuggling, increasing its population by breeding it in captivity and, if possible, introducing it on to a neighbouring island. Introduction up of two parts linked by a narrow strip of land. This island, with 27 Melanesian inhabi- The Ouvea parakeet Eunymphicus cornutus tants per sq km, has a population density uvaeensis and the horned parakeet E. c. cornu- three times that of the rest of the Loyalty tus are the only two members of an endemic Islands (CTRDP, 1987; Mathieu-Daude, 1989). New Caledonian genus. The genus is related The parakeet's distribution is restricted to to Platycercus of Australia and Cyanoramphus Ouvea. It appears to have never colonized of New Zealand by its form and colouring, neighbouring islands despite their proximity which is mostly green with a red forehead and (45 km). -
The Extinction of the Carolina Parakeet and Multiple Dimensions of Global Parrot Biodiversity Kevin R
University of Connecticut OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 5-4-2017 The Extinction of the Carolina Parakeet and Multiple Dimensions of Global Parrot Biodiversity Kevin R. Burgio University of Connecticut - Storrs, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Burgio, Kevin R., "The Extinction of the Carolina Parakeet and Multiple Dimensions of Global Parrot Biodiversity" (2017). Doctoral Dissertations. 1422. https://opencommons.uconn.edu/dissertations/1422 The Extinction of the Carolina Parakeet and Multiple Dimensions of Global Parrot Biodiversity Kevin Roy Burgio, PhD University of Connecticut, 2017 The study of the ecology of a species has traditionally ceased when that species goes extinct, despite the benefit to current and future generations of potential findings. We used the Carolina parakeet to develop a framework investigating the distributional limits, migratory habits, and extinction process as a means to recover important information. We developed a comprehensive database of every known occurrence of this iconic species. Using a combination of environmental niche modeling and extinction estimating analyses, our results demonstrate that the Carolina parakeet’s range was smaller than previously believed, the eastern and western subspecies occupied different niches with broad geographic separation, and that the western subspecies was a seasonal migrant while the eastern subspecies was not. We also found that it was likely habitat loss played a major role in their extinction. Our study highlights the importance of collecting occurrence data of extinct species and provides a framework for further investigations of other extinct species. Moreover, the recovery of lost autecological knowledge could benefit the conservation of other species currently in decline. -
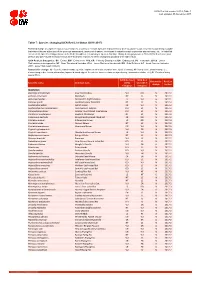
Table 7 Last Updated: 05 December 2017
IUCN Red List version 2017-3: Table 7 Last Updated: 05 December 2017 Table 7: Species changing IUCN Red List Status (2016-2017) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2016 (IUCN Red List version 2016.3) and 2017 (IUCN Red List version 2017-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2016) List (2017) change version Category Category MAMMALS Allactaga tetradactyla Four-toed Jerboa VU DD N 2017-2 Antilope cervicapra Blackbuck NT LC N 2017-2