Functional and Transcriptional Characterisation of Synovial Fibroblasts in Early Inflammatory Arthritis and Established Rheumatoid Arthritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sorting Nexin 27 Regulates the Lysosomal Degradation of Aquaporin-2 Protein in the Kidney Collecting Duct
cells Article Sorting Nexin 27 Regulates the Lysosomal Degradation of Aquaporin-2 Protein in the Kidney Collecting Duct Hyo-Jung Choi 1,2, Hyo-Ju Jang 1,3, Euijung Park 1,3, Stine Julie Tingskov 4, Rikke Nørregaard 4, Hyun Jun Jung 5 and Tae-Hwan Kwon 1,3,* 1 Department of Biochemistry and Cell Biology, School of Medicine, Kyungpook National University, Taegu 41944, Korea; [email protected] (H.-J.C.); [email protected] (H.-J.J.); [email protected] (E.P.) 2 New Drug Development Center, Daegu-Gyeongbuk Medical Innovation Foundation, Taegu 41061, Korea 3 BK21 Plus KNU Biomedical Convergence Program, Department of Biomedical Science, School of Medicine, Kyungpook National University, Taegu 41944, Korea 4 Department of Clinical Medicine, Aarhus University, Aarhus 8200, Denmark; [email protected] (S.J.T.); [email protected] (R.N.) 5 Division of Nephrology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; [email protected] * Correspondence: [email protected]; Tel.: +82-53-420-4825; Fax: +82-53-422-1466 Received: 30 March 2020; Accepted: 11 May 2020; Published: 13 May 2020 Abstract: Sorting nexin 27 (SNX27), a PDZ (Postsynaptic density-95/Discs large/Zonula occludens 1) domain-containing protein, cooperates with a retromer complex, which regulates intracellular trafficking and the abundance of membrane proteins. Since the carboxyl terminus of aquaporin-2 (AQP2c) has a class I PDZ-interacting motif (X-T/S-X-F), the role of SNX27 in the regulation of AQP2 was studied. Co-immunoprecipitation assay of the rat kidney demonstrated an interaction of SNX27 with AQP2. -

Sorting Nexin-27 Regulates AMPA Receptor Trafficking Through The
RESEARCH ARTICLE Sorting nexin-27 regulates AMPA receptor trafficking through the synaptic adhesion protein LRFN2 Kirsty J McMillan1†*, Paul J Banks2†, Francesca LN Hellel1, Ruth E Carmichael1, Thomas Clairfeuille3, Ashley J Evans1, Kate J Heesom4, Philip Lewis4, Brett M Collins3, Zafar I Bashir2, Jeremy M Henley1, Kevin A Wilkinson1*, Peter J Cullen1* 1School of Biochemistry, University of Bristol, Bristol, United Kingdom; 2School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, United Kingdom; 3Institute for Molecular Bioscience, The University of Queensland, Queensland, Australia; 4Proteomics facility, School of Biochemistry, University of Bristol, Bristol, United Kingdom Abstract The endosome-associated cargo adaptor sorting nexin-27 (SNX27) is linked to various neuropathologies through sorting of integral proteins to the synaptic surface, most notably AMPA receptors. To provide a broader view of SNX27-associated pathologies, we performed proteomics in rat primary neurons to identify SNX27-dependent cargoes, and identified proteins linked to excitotoxicity, epilepsy, intellectual disabilities, and working memory deficits. Focusing on the *For correspondence: synaptic adhesion molecule LRFN2, we established that SNX27 binds to LRFN2 and regulates its [email protected] endosomal sorting. Furthermore, LRFN2 associates with AMPA receptors and knockdown of (KJMM); LRFN2 results in decreased surface AMPA receptor expression, reduced synaptic activity, and [email protected] attenuated hippocampal long-term potentiation. Overall, our study provides an additional (KAW); mechanism by which SNX27 can control AMPA receptor-mediated synaptic transmission and [email protected] (PJC) plasticity indirectly through the sorting of LRFN2 and offers molecular insight into the perturbed †These authors contributed function of SNX27 and LRFN2 in a range of neurological conditions. -

Bone Morphogenetic Protein-4 Affects Both Trophoblast and Non-Trophoblast Lineage-Associated Gene Expression in Human Embryonic Stem Cells
Vol.2, No.4, 163-175 (2012) Stem Cell Discovery http://dx.doi.org/10.4236/scd.2012.24021 Bone morphogenetic protein-4 affects both trophoblast and non-trophoblast lineage-associated gene expression in human embryonic stem cells Margaret L. Shirley1,2*, Alison Venable1*, Raj R. Rao3, Nolan L. Boyd4, Steven L. Stice1,5,6, David Puett1#, Prema Narayan7# 1Department of Biochemistry and Molecular Biology, University of Georgia, Athens, USA; #Corresponding Author: [email protected] 2Department of Psychiatry, University of California, San Francisco, USA 3Department of Chemical and Life Science Engineering, School of Engineering, Virginia Commonwealth University, Richmond, USA 4Cardiovascular Innovation Institute, University of Louisville, Louisville, USA 5Regenerative Bioscience Center, University of Georgia, Athens, USA 6Department of Animal and Dairy Sciences, University of Georgia, Athens, USA 7Department of Physiology, Southern Illinois University School of Medicine, Carbondale, USA; #Corresponding Author: [email protected] Received 5 May 2012; revised 4 June 2012; accepted 1 July 2012 ABSTRACT cells were obtained. Gene expression by EB was characterized by an up-regulation of a num- Human embryonic stem cells (hESC) can be in- ber of genes associated with trophoblast, ecto- duced to differentiate to trophoblast by bone derm, endoderm, and mesoderm, and the pro- morphogenetic proteins (BMPs) and by aggre- duction of hCG and progesterone confirmed that gation to form embryoid bodies (EB), but there trophoblast-like cells were formed. These re- are many differences and controversies regard- sults suggest that, in the presence of FGF-2, ing the nature of the differentiated cells. Our BG02 cells respond to BMP4 to yield tropho- goals herein were to determine if BG02 cells form trophoblast-like cells (a) in the presence of blast-like cells, which are also obtained upon EB BMP4-plus-basic fibroblast growth factor (FGF-2) formation. -

Silencing of SNX1 by Sirna Stimulates the Ligand-Induced Endocytosis of EGFR and Increases EGFR Phosphorylation in Gefitinib-Resistant Human Lung Cancer Cell Lines
1520 INTERNATIONAL JOURNAL OF ONCOLOGY 41: 1520-1530, 2012 Silencing of SNX1 by siRNA stimulates the ligand-induced endocytosis of EGFR and increases EGFR phosphorylation in gefitinib-resistant human lung cancer cell lines YUKIO NISHIMURA1, SOICHI TAKIGUCHI2, KIYOKO YOSHIOKA3, YUSAKU NAKABEPPU4 and KAZUYUKI ITOH3 1Division of Pharmaceutical Cell Biology, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka 812-8582; 2Institute for Clinical Research, National Kyushu Cancer Center, Fukuoka 811-1395; 3Department of Biology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511; 4Division of Neurofunctional Genomics, Department of Immunobiology and Neuroscience, Medical Institute of Bioregulation, Kyushu University, Fukuoka 812-8582, Japan Received May 8, 2012; Accepted July 6, 2012 DOI: 10.3892/ijo.2012.1578 Abstract. Gefitinib is known to suppress the activation of delivery of pEGFR from early endosomes to late endosomes. EGFR signaling, which is required for cell survival and Further, western blot analysis revealed that silencing of SNX1 proliferation in non-small cell lung cancer (NSCLC) cell expression by siRNA in the gefitinib-resistant cells leads to lines. We previously demonstrated that the gefitinib-sensitive an accelerated degradation of EGFR along with a dramatic NSCLC cell line PC9 shows efficient ligand-induced endo- increase in the amounts of pEGFR after EGF stimulation. cytosis of phosphorylated EGFR (pEGFR). In contrast, the Based on these findings, we suggest that SNX1 is involved in gefitinib-resistant NSCLC cell lines QG56 and A549 showed the negative regulation of ligand-induced EGFR phosphory- internalized pEGFR accumulation in the aggregated early lation and mediates EGFR/pEGFR trafficking out of early endosomes, and this was associated with SNX1, a protein that endosomes for targeting to late endosomes/lysosomes via the interacts with and enhances the degradation of EGFR upon early/late endocytic pathway in human lung cancer cells. -
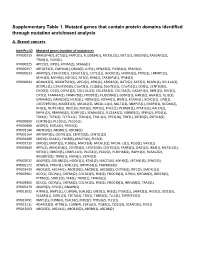
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

Overview Gene List Target Scan Vs DIANA Group a Group B Group A
Overview Gene list Target scan vs DIANA Group A Group B Group A hsa-miR-181a hsa-miR-323 hsa-miR-326 Target scan Diana microT Overlap Target scan Diana microT Overlap Target scan SEPT3 SEPT3 SEPT3 SEPT7 ADARB1 HPCAL4 ABHD2 ABL2 ABHD13 ACVR2A ADCYAP1R1 AKAP13 PDPK1 ACRBP ACAN ABI1 ADAMTS1 ALAD APOBR ACVRL1 ACCN2 ABLIM1 ADAMTSL1 ANKRD52 ATXN1 ADAM19 ACER3 ACSL1 AKAP7 ARID2 C18orf23,RNF165 ADAM33 ACVR2A ACTN2 ANKRD43 ARL3 C20orf29 ADAMTS2 ADAMTS1 ACVR2A AP1S3 ARRB1 CACNG4 AHCYL2 ADAMTS18 ACVR2B ARID2 BBC3 CCNJL ALOX15B ADAMTS5 ADAM11 ATP11A BTG1 CYP2E1 ANK1 ADAMTSL1 ADAM22 ATXN1 C18orf62 GNB1L ANKS6 ADARB1 ADAMTS1 B4GALT1 C1orf21 GPR61 APBA1 AFAP1 ADAMTS6 BAG4 CADM4 GTSE1 ARCN1 AFTPH ADAMTSL1 BAI3 CALML4 HPCAL4 ARHGEF37 AK3 ADCY9 BNC2 CAPN6 KIAA0152 ARID3B AKAP7 ADRBK1 BRD1 CBFA2T2 KIF1A ARL8A ANAPC16 AFF2 BRWD1 CEBPA MACF1 ATP2B2 ANK1 AHCTF1,AHCTF1PBTBD3 CHD1 MYO1D ATP6V1G2 ANKRD12 AKAP2,PALM2 C13orf23 CIT PCNT AUP1 ANKRD33B AKAP6 C14orf43 CLASP2 PDPK1 BCL2L2 ANKRD43 AKAP7 CAPRIN1 CLCN5 PLEKHG4B BHLHE40 ANKRD44 AKAP9 CARM1 CLIP3 PPARA BTBD3 ANKRD52 AKT3 CBX4 COL5A2 PRB1,PRB2,PRB4 BTRC AP1S3 ALG9 CCDC117 CTNS PTPRT C10orf26 APBA1 ANKRD13C CCNJ DCTN4 PYCR1 C14orf1 APLP2 ANKRD20B CDH13 DCUN1D4 RAPGEF1 C16orf45 APOO ANKRD43 CDON DDB1 SRCAP C16orf54 ARID2 ANKRD50 CDYL DDX39B TMEM63C C1orf106 ARL3 AP1G1 CEP350 DIP2C C1orf27 ARRDC3 AP1S3 CHD7 DNAJB3 C22orf29 ATF7 API5 CHIC1 EEPD1 C9orf3 ATG2B ARFGEF2 CLIP1 EIF2C1 CACNA1E ATG7 ARHGAP12 CNOT6L ELFN2 CAPN12 ATP11A ARHGAP26 CNR1 ELK1 CASKIN1 ATP2B3 ARHGAP29 CNTN4 FAM172A CBFA2T3 ATP8B2 ARHGEF3 CNTNAP2 -

A Targeted Rnai Screen Identifies Endocytic Trafficking Factors That
Diabetes Volume 67, March 2018 385 A Targeted RNAi Screen Identifies Endocytic Trafficking Factors That Control GLP-1 Receptor Signaling in Pancreatic b-Cells Teresa Buenaventura,1 Nisha Kanda,1 Phoebe C. Douzenis,1 Ben Jones,2 Stephen R. Bloom,2 Pauline Chabosseau,1 Ivan R. Corrêa Jr.,3 Domenico Bosco,4 Lorenzo Piemonti,5,6 Piero Marchetti,7 Paul R. Johnson,8 A.M. James Shapiro,9 Guy A. Rutter,1 and Alejandra Tomas1 Diabetes 2018;67:385–399 | https://doi.org/10.2337/db17-0639 The glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) is a key binding, GLP-1Rs signal through stimulatory G (Gs) pro- target for type 2 diabetes (T2D) treatment. Because endocytic teins to raise intracellular cAMP levels and modulate insulin trafficking of agonist-bound receptors is one of the most secretion from pancreatic b-cells. important routes for regulation of receptor signaling, a better As with other G protein–coupled receptors (GPCRs), SIGNAL TRANSDUCTION understanding of this process may facilitate the develop- GLP-1R signaling is tightly regulated through endocytic ment of new T2D therapeutic strategies. Here, we screened trafficking (2). Activated GLP-1Rs are rapidly internalized – 29 proteins with known functions in G protein coupled recep- and recycled to the plasma membrane or distributed through- fi tor traf cking for their role in GLP-1R potentiation of insulin out endocytic compartments (3). Despite the pivotal role b fi secretion in pancreatic -cells. We identify ve (clathrin, of trafficking in GPCR signaling, neither the molecular dynamin1, AP2, sorting nexins [SNX] SNX27, and SNX1) details nor the key factors responsible for GLP-1R endo- that increase and four (huntingtin-interacting protein 1 cytosis and postendocytic sorting have been thoroughly [HIP1], HIP14, GASP-1, and Nedd4) that decrease insulin investigated. -

Identifying the Risky SNP of Osteoporosis with ID3-PEP Decision Tree Algorithm
Hindawi Complexity Volume 2017, Article ID 9194801, 8 pages https://doi.org/10.1155/2017/9194801 Research Article Identifying the Risky SNP of Osteoporosis with ID3-PEP Decision Tree Algorithm Jincai Yang,1 Huichao Gu,1 Xingpeng Jiang,1 Qingyang Huang,2 Xiaohua Hu,1 and Xianjun Shen1 1 School of Computer Science, Central China Normal University, Wuhan 430079, China 2School of Life Science, Central China Normal University, Wuhan 430079, China Correspondence should be addressed to Jincai Yang; [email protected] Received 31 March 2017; Revised 26 May 2017; Accepted 8 June 2017; Published 7 August 2017 Academic Editor: Fang-Xiang Wu Copyright © 2017 Jincai Yang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In the past 20 years, much progress has been made on the genetic analysis of osteoporosis. A number of genes and SNPs associated with osteoporosis have been found through GWAS method. In this paper, we intend to identify the suspected risky SNPs of osteoporosis with computational methods based on the known osteoporosis GWAS-associated SNPs. The process includes two steps. Firstly, we decided whether the genes associated with the suspected risky SNPs are associated with osteoporosis by using random walk algorithm on the PPI network of osteoporosis GWAS-associated genes and the genes associated with the suspected risky SNPs. In order to solve the overfitting problem in ID3 decision tree algorithm, we then classified the SNPs with positive results based on their features of position and function through a simplified classification decision tree which was constructed by ID3 decision tree algorithm with PEP (Pessimistic-Error Pruning). -

SNX1 Coats on Sorting Endosomes 1745 Immobilized on Glutathione-Agarose in 200 Μl Reactions
RESEARCH ARTICLE 1743 Self-assembly and binding of a sorting nexin to sorting endosomes Richard C. Kurten*, Anthony D. Eddington, Parag Chowdhury, Richard D. Smith, April D. Davidson and Brian B. Shank Department of Physiology and Biophysics and Arkansas Cancer Research Center, University of Arkansas for Medical Sciences, 4301 West Markham Street, Little Rock, Arkansas 72205-0750, USA *Author for correspondence (e-mail: [email protected]) Accepted 20 February 2001 Journal of Cell Science 114, 1743-1756 © The Company of Biologists Ltd SUMMARY The fate of endocytosed membrane proteins and luminal cytoplasmic and early/sorting endosome-bound pools of contents is determined by a materials processing system in green fluorescent protein-SNX1. Fluorescence resonance sorting endosomes. Endosomal retention is a mechanism energy transfer indicated that spectral variants of green that traps specific proteins within this compartment, and fluorescent protein-SNX1 were oligomeric in vivo. In cell thereby prevents their recycling. We report that a sorting extracts, these green fluorescent protein-SNX1 oligomers nexin SNX1, a candidate endosomal retention protein, self- corresponded to tetrameric and larger complexes of green assembles in vitro and in vivo, and has this property in fluorescent protein-SNX1. Using video microscopy, we common with its yeast homologue Vps5p. A comparison of observed small vesicle docking and tubule budding from SNX1 expressed in bacterial and in mammalian systems large green fluorescent protein-SNX1 coated endosomes, and analyzed by size-exclusion chromatography indicates which are features consistent with their role as sorting that in cytosol SNX1 tetramers are part of a larger complex endosomes. with additional proteins. -

An Essential Role for SNX1 in Lysosomal Sorting of Protease
Molecular Biology of the Cell Vol. 17, 1228–1238, March 2006 An Essential Role for SNX1 in Lysosomal Sorting of Protease-activated Receptor-1: Evidence for Retromer-, Hrs-, and Tsg101-independent Functions of Sorting Nexins Anuradha Gullapalli,* Breann L. Wolfe,* Courtney T. Griffin,† Terry Magnuson,† and JoAnn Trejo*‡ Departments of *Pharmacology, ‡Cell and Developmental Biology, and †Genetics, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7365 Submitted September 28, 2005; Revised December 16, 2005; Accepted January 3, 2006 Monitoring Editor: Sandra Schmid Sorting nexin 1 (SNX1) and SNX2 are the mammalian homologues of the yeast Vps5p retromer component that functions in endosome-to-Golgi trafficking. SNX1 is also implicated in endosome-to-lysosome sorting of cell surface receptors, although its requirement in this process remains to be determined. To assess SNX1 function in endocytic sorting of protease-activated receptor-1 (PAR1), we used siRNA to deplete HeLa cells of endogenous SNX1 protein. PAR1, a G-protein-coupled receptor, is proteolytically activated by thrombin, internalized, sorted predominantly to lysosomes, and efficiently degraded. Strikingly, depletion of endogenous SNX1 by siRNA markedly inhibited agonist-induced PAR1 degradation, whereas expression of a SNX1 siRNA-resistant mutant protein restored agonist-promoted PAR1 degradation in cells lacking endogenous SNX1, indicating that SNX1 is necessary for lysosomal degradation of PAR1. SNX1 is known to interact with components of the mammalian retromer complex and Hrs, an early endosomal membrane-associated protein. However, activated PAR1 degradation was not affected in cells depleted of retromer Vps26/Vps35 subunits, Hrs or Tsg101, an Hrs-interacting protein. -

Molecular Regulation in Dopaminergic Neuron Development
International Journal of Molecular Sciences Review Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration Floriana Volpicelli 1 , Carla Perrone-Capano 1,2 , Gian Carlo Bellenchi 2,3, Luca Colucci-D’Amato 4,* and Umberto di Porzio 2 1 Department of Pharmacy, University of Naples Federico II, 80131 Naples, Italy; fl[email protected] (F.V.); [email protected] (C.P.C.) 2 Institute of Genetics and Biophysics “Adriano Buzzati Traverso”, CNR, 80131 Rome, Italy; [email protected] (G.C.B.); [email protected] (U.d.P.) 3 Department of Systems Medicine, University of Rome Tor Vergata, 00133 Rome, Italy 4 Department of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania “Luigi Vanvitelli”, 81100 Caserta, Italy * Correspondence: [email protected]; Tel.: +39-0823-274577 Received: 28 April 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The relatively few dopaminergic neurons in the mammalian brain are mostly located in the midbrain and regulate many important neural functions, including motor integration, cognition, emotive behaviors and reward. Therefore, alteration of their function or degeneration leads to severe neurological and neuropsychiatric diseases. Unraveling the mechanisms of midbrain dopaminergic (mDA) phenotype induction and maturation and elucidating the role of the gene network involved in the development and maintenance of these neurons is of pivotal importance to rescue or substitute these cells in order to restore dopaminergic functions. Recently, in addition to morphogens and transcription factors, microRNAs have been identified as critical players to confer mDA identity. The elucidation of the gene network involved in mDA neuron development and function will be crucial to identify early changes of mDA neurons that occur in pre-symptomatic pathological conditions, such as Parkinson’s disease. -

Molecular Basis for SNX-BAR-Mediated Assembly Of
The EMBO Journal (2012) 31, 4466–4480 | & 2012 European Molecular Biology Organization | Some Rights Reserved 0261-4189/12 www.embojournal.org TTHEH E EEMBOMBO JJOURNALOURN AL Molecular basis for SNX-BAR-mediated assembly EMBO of distinct endosomal sorting tubules open Jan RT van Weering1,5, Introduction Richard B Sessions1, Colin J Traer1, 2,6 3,7 Endosomal sorting is an essential process for maintaining Daniel P Kloer , Vikram K Bhatia , cellular homeostasis with deregulated sorting underlying a Dimitrios Stamou3, Sven R Carlsson4, 2 1, variety of pathologies, including neurodegenerative diseases James H Hurley and Peter J Cullen * and cancer (Huotari and Helenius, 2011). Endosomal sorting 1The Henry Wellcome Integrated Signalling Laboratories, School of is achieved in the tubular endosomal network (TEN), a Biochemistry, University of Bristol, Bristol, UK, 2Laboratory of complex arrangement of tubular and vesicular structures Molecular Biology, National Institute of Diabetes and Digestive and that surrounds the endosomal vacuole (Wall et al, 1980). Kidney Diseases, National Institutes of Health, Bethesda, MD, USA, 3Bio-Nanotechnology and Nanomedicine Laboratory, Department of These tubular/vesicular membrane profiles constitute Chemistry and Nano-Science Center, Lundbeck Foundation Center molecularly distinct sorting platforms for the recycling Biomembranes in Nanomedicine, University of Copenhagen, Copenhagen, of proteins back to the plasma membrane or to the 4 Denmark and Department of Medical Biochemistry and Biophysics, trans-Golgi network (TGN; Geuze et al, 1983). How such a Umea University, Umea˚, Sweden complex tubular–vesicular arrangement is generated and how individual tubules maintain their molecular identities Sorting nexins (SNXs) are regulators of endosomal sorting. during the processes of protein sorting and membrane traf- For the SNX-BAR subgroup, a Bin/Amphiphysin/Rvs ficking remains, on the whole, unclear.