The Effect of Eye Protection on SARS-Cov-2 Transmission: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
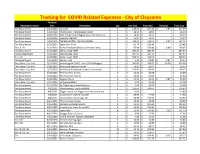
Claycomo Master List Updated 5.18.21.Xlsx
Tracking for COVID Related Expenses - City of Claycomo Purchase Department Name Date Description Qty Unit Cost Extended Shipping Total Cost Fire Department 3/12/2020Cavicide 2$ 128.56 $ 257.12 $ 0.87 $ 257.99 Fire Department 3/18/2020 Thermoscan Thermometer Covers 1$ 18.09 $ 18.09 $ 18.09 Fire Department 3/18/2020 ADC Temple Touch Digital Fever Thermometer 3$ 14.41 $ 43.23 $ 43.23 Fire Department 3/18/2020 Lysol Disinfectant 1$ 45.94 $ 45.94 $ 45.94 Fire Department 3/18/2020 THERMOMETER, EAR PRO 6000W 1$ 240.64 $ 240.64 $ 0.87 $ 241.51 Fire Department 3/23/2020 Super Sani-Cloth 2$ 61.74 $ 123.48 $ 123.48 FD, CH, PD 3/23/2020 Remon Newest 60W UV Germicidal Lamp 2$ 69.99 $ 139.98 $ 8.99 $ 148.97 Fire Department 3/23/2020 Dell Latitude 7290 1$ 487.47 $ 487.47 $ 487.47 Police Department 3/24/2020 Dell Latitude 7290 1$ 487.47 $ 487.47 $ 487.47 City Hall 3/24/2020 Dell Latitude 7290 1$ 558.57 $ 558.57 $ 558.57 Fire Department 3/25/2020Gloves - XLG 4$ 5.45 $ 21.80 $ 0.87 $ 22.67 Fire, Police, City Hall 3/25/2020 VectorFog Go Z200 5 Liter ULV Cold Fogger 1$ 595.00 $ 595.00 $ 35.38 $ 630.38 Fire, Police, City Hall 3/26/2020 3x silicone keyboard cover 3$ 8.99 $ 26.97 $ 26.97 Fire, Police, City Hall 3/27/2020 Mediclean Germicidal Cleaner Concentrate 1$ 174.95 $ 174.95 $ 174.95 Fire Department 3/29/2020 Thermometer Covers 2$ 12.49 $ 24.98 $ 24.98 Fire Department 3/29/2020 Thermometer Covers 2$ 13.80 $ 27.60 $ 27.60 Fire Department 3/30/2020 Surgical Masks 3$ 11.51 $ 34.53 $ 0.87 $ 35.40 Fire, Police, City Hall 3/31/2020 UV Lamp Holders 2$ 9.99 $ -

Whitepaper Head Mounted Displays & Data Glasses Applications and Systems
Whitepaper Head Mounted Displays & Data Glasses Applications and Systems Dr.-Ing. Dipl.-Kfm. Christoph Runde Virtual Dimension Center (VDC) Fellbach Auberlenstr. 13 70736 Fellbach www.vdc-fellbach.de © Competence Centre for Virtual Reality and Cooperative Engineering w. V. – Virtual Dimension Center (VDC) System classes Application fields Directions of development Summary Content . System classes Head Mounted Display (HMD) – Video glasses – Data glasses . Simulator disease / Cyber Sickness . Application fields HMDs: interior inspections, training, virtual hedging engineering / ergonomics . Application fields data glasses: process support, teleservice, consistency checks, collaboration . Directions of development: technical specifications, (eye) tracking, retinal displays, light field technology, imaging depth sensors . Application preconditions information & integration (human, IT, processes) . Final remark 2 SystemSystem classes classes Application fields Directions of development Summary Head Mounted Displays (HMDs) – Overview . 1961: first HMD on market . 1965: 3D-tracked HMD by Ivan Sutherland . Since the 1970s a significant number of HMDs is applied in the military sector (training, additional display) Table: Important HMD- projects since the 1970s [Quelle: Li, Hua et. al.: Review and analysis of avionic helmet-mounted displays. In : Op-tical Engineering 52(11), 110901, Novembre2013] 3 SystemSystem classes classes Application fields Directions of development Summary Classification HMD – Video glasses – Data glasses Head Mounted Display -

Assessing Next-Generation Construction Helmets
Assessing Next-Generation May 2018 Construction Helmets The KASK - Zenith and Superplasma Helmets Skanska’s Advancement In Head Protection Pilot Program Current Status Currently, Skanska uses the MSA V-Gard as its primary choice of hard hat head protection. This hard hat was introduced to the US 56 years ago. The V Guard (and all other regular styles of hard hats) primarily protects against objects striking the crown of a person’s head. This type of head protection offers minimal, if any protection to the front, rear and sides of the head. Comparatively, head protection such as the KASK helmet (not a hard hat) offers much more protection. Although it is listed as Type I head protection, it has passed ANSI Type II impact tests to the front, rear and sides. The Need for Change Skanska USA is investigating the advancements of head protection for employees working on its projects. Helmets being piloted on our projects include products from KASK, MSA Nexius, and 3M X5000. There are many reasons why we are looking to improve the current head protection. The primary reason is the fact that head injuries of all classifications still occur on our projects. These injuries vary on the spectrum of severity, from minimal in nature to catastrophic. Plain and simple: changes in head protection are needed in our industry. Skanska is looking to champion these efforts through an in-depth evaluation of different types of head protection, including the KASK helmets described in this white paper. A thorough list of the reasons for change include: • During any fall incident (slips, trips and falls from the same level, falls from ladders, falls from wall forms, etc.) the current hard hat is prone to fall off a worker’s head prior to impact because of the tendency for the head to “snap backwards,” leaving the head exposed when protection is most needed. -

Tactical Eyewear Protection Equipment Assessment Report
Tactical Eyewear Protection Equipment Assessment Report May 2020 Approved for Public Release SAVER-T-R-21 The Tactical Eyewear Protection Equipment Assessment Report was funded under Financial Transaction FTLF- 19-00009 from the U.S. Department of Homeland Security, Science and Technology Directorate. The views and opinions of authors expressed herein do not necessarily reflect those of the U.S. Government. Reference herein to any specific commercial products, processes, or services by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the U.S. Government. The information and statements contained herein shall not be used for the purposes of advertising, nor to imply the endorsement or recommendation of the U.S. Government. With respect to documentation contained herein, neither the U.S. Government nor any of its employees make any warranty, express or implied, including but not limited to the warranties of merchantability and fitness for a particular purpose. Further, neither the U.S. Government nor any of its employees assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed; nor do they represent that its use would not infringe privately owned rights. The cover photo and images included herein were provided by the National Urban Security Technology Laboratory, unless otherwise noted. Approved for Public Release ii FOREWORD The U.S. Department of Homeland Security (DHS) established the System Assessment and Validation for Emergency Responders (SAVER) Program to assist emergency responders making procurement decisions. Located within the Science and Technology Directorate (S&T) of DHS, the SAVER Program conducts objective assessments and validations on commercially available equipment and systems and develops knowledge products that provide relevant equipment information to the emergency responder community. -

Using Smart Glasses and Augmented Reality Head-Mounted Displays To
Using smart glasses and augmented reality head-mounted displays to drive supply chain innovation A series exploring Industry 4.0 technologies and their potential impact for enabling digital supply networks in manufacturing Using smart glasses and augmented reality head-mounted displays to drive supply chain innovation Contents What are smart glasses and augmented reality HMDs? 4 Benefits of smart glasses and AR in the supply chain 6 Criteria for evaluation and adoption 8 Key applications for smart glasses in your supply chain 10 Getting started with smart glasses and augmented reality devices 11 Key Deloitte contacts in smart glasses and augmented reality 12 02 Using smart glasses and augmented reality head-mounted displays to drive supply chain innovation Do you need smart glasses in your supply chain? Smart glasses are already being used to improve quality, standardize workflows, accelerate decision making, and enhance communication in logistics, manufacturing, and maintenance operations throughout the supply chain. Of interest because: A trend in more universal, data-driven decision making is evolving just as leaps in hardware and software capability have led to smaller, more ergonomic devices. Could improve your supply chain by: Boosting labor effectiveness and productivity; reducing quality defects and rework; improving workflow standardization; enhancing workforce collaboration; improving safety. Why not? The technology is still rapidly evolving and has not yet reached operational maturity. Companies will have to understand how they will leverage data for critical use cases and should expect difficult integration challenges. Deloitte recommends: Evaluate the business case for smart glasses and augmented reality in your supply chain and pilot on a small number of critical use cases. -

Tobii Pro Glasses 2 Helmet Edition Product Description
Tobii Pro Glasses 2 Helmet Edition Product Description Head Unit (Firmware version 1.25.3) Recording Unit Prescription Lenses Controller Software Product Description Tobii Pro Glasses 2 Helmet Edition Version 1.0.1 08/2018 All rights reserved. Copyright © Tobii AB (publ) The information contained in this document is proprietary to Tobii AB. Any reproduction in part or whole without prior writ- ten authorization by Tobii AB is prohibited. Products that are referred to in this document may be either trademarks and/or registered trademarks of the respective owners. The publisher and the author make no claim to these trademarks. While every precaution has been taken in the preparation of this document, the publisher and the author assume no re- sponsibility for errors or omissions, or for damages resulting from the use of information contained in this document or from the use of programs and source code that may accom- pany it. In no event shall the publisher and the author be liable for any loss of profit or any other commercial damage caused or alleged to have been caused directly or indirectly by this document. Content subject to change without notice. Please check Tobii web site www.tobiipro.com for updated versions of this document. Table of Contents 1 Introduction.................................................................................................................................... 1 2 System Overview .......................................................................................................................... -

Regular Glasses Aren't Safety Glasses
10-minute safety talk Regular eyeglasses aren’t safety glasses Wearing prescription glasses into a hazardous work environment where safety glasses are required might seem safe. In reality, regular glasses don’t protect a person’s eyes as well as rated safety glasses. Key takeaways The American National Standards Institute (ANSI) develops safety specific requirements for • Regular glass personal protective equipment (PPE). To be stamped with an appropriate ANSI rating, safety frames and lenses can shatter and send glasses must pass many tests. ANSI Z87 is the rating for basic eye protection. The ANSI Z87+ shards into eyes. rating means the glasses can withstand significant impact, like the blast of a pellet gun. For chemicals, dusts, and various lights, you may need different PPE, such as goggles, with a • Unless ANSI rated, regular glasses are different ANSI rating. not meant to protect Prescription glasses are made to correct vision, not for personal protection. Lenses provided eyes from debris. with prescription glasses may shatter and the frames do not provide side impact protection. The • Safety rated glasses good news? Safety glasses with ANSI ratings can be made with corrective lenses. They may look will have an ANSI just like standard reading glasses, but the ANSI rating stamped on the bow of the frame means rating stamped on the bow of the that both the frames and the lenses meet the safety standard. frame. The ANSI Z87 rating stamp refers to protection against impact. • If safety rated glasses are worn without side shields, they are not considered safety glasses. Take action (Complete one or more activities as a team) A. -

Eyewear Frames and Eyewear
What Every Member of the Trade Community Should Know About: Eyewear Frames and Eyewear AN INFORMED COMPLIANCE PUBLICATION FEBRUARY 2012 Eyewear Frames and Eyewear February 2012 NOTICE: This publication is intended to provide guidance and information to the trade community. It reflects the position on or interpretation of the applicable laws or regulations by U.S. Customs and Border Protection (CBP) as of the date of publication, which is shown on the front cover. It does not in any way replace or supersede those laws or regulations. Only the latest official version of the laws or regulations is authoritative. Publication History First Published: January 2008 Revised April 2009 Revised January 2011 Reviewed with No Changes February 2012 PRINTING NOTE: This publication was designed for electronic distribution via the CBP website (http://www.cbp.gov) and is being distributed in a variety of formats. It was originally set up in Microsoft Word 2003®. Pagination and margins in downloaded versions may vary depending upon which word processor or printer you use. If you wish to maintain the original settings, you may wish to download the .pdf version, which can then be printed using the freely available Adobe Acrobat Reader®. 2 Eyewear Frames and Eyewear February 2012 PREFACE On December 8, 1993, Title VI of the North American Free Trade Agreement Implementation Act (Pub. L. 103-182, 107 Stat. 2057), also known as the Customs Modernization or “Mod” Act, became effective. These provisions amended many sections of the Tariff Act of 1930 and related laws. Two new concepts that emerge from the Mod Act are “informed compliance” and “shared responsibility,” which are premised on the idea that in order to maximize voluntary compliance with laws and regulations of U.S. -

Culture of Azerbaijan
Administrative Department of the President of the Republic of Azerbaijan P R E S I D E N T I A L L I B R A R Y CULTURE OF AZERBAIJAN CONTENTS I. GENERAL INFORMATION............................................................................................................. 3 II. MATERIAL CULTURE ................................................................................................................... 5 III. MUSIC, NATIONAL MUSIC INSTRUMENTS .......................................................................... 7 Musical instruments ............................................................................................................................... 7 Performing Arts ....................................................................................................................................... 9 Percussion instruments ........................................................................................................................... 9 Wind instruments .................................................................................................................................. 12 Mugham as a national music of Azerbaijan ...................................................................................... 25 IV. FOLKLORE SONGS ..................................................................................................................... 26 Ashiqs of Azerbaijan ............................................................................................................................ 27 V. THEATRE, -

Most Face Shields, Goggles, and Safety Glasses Can Be Cleaned/Disinfected and Re-Used
COVID-19: Cleaning and Disinfection of Face Shields, Goggles and Safety Glasses Most face shields, goggles, and safety glasses can be cleaned/disinfected and re-used. In general, eye protection should be dedicated for individual use and should not be shared. Eye protection should be cleaned and disinfected between uses. Cleaning products and PPE required Sink or running water, soap (e.g. dish soap), disinfectant wipes approved for use against COVID-19 (Health Canada DIN number), and disposable gloves (nitrile, vinyl). Inspect eye protection Goggles, safety glasses, and face shields without foam: Inspect the eye protection for damage (e.g. broken bands or straps, scratches on visor or lens). If the eye protection appears damaged or compromised, DO NOT REUSE. Face shields with visor and foam for forehead comfort: Inspect the face shield for damage. If the foam piece of the face shield is visibly soiled or the face shield appears damaged or compromised, DO NOT REUSE. Cleaning and disinfection Eye protection should be cleaned following manufacturer’s instructions. Review the manufacturer’s instructions for the type of eye protection you are using and for the cleaner/disinfectant. If instructions are not available, use the following steps: 1. Place eye protection on a clean surface. 2. Perform hand hygiene. 3. Put on a new pair of disposable gloves (if indicated by disinfectant label). 4. Clean: Using a clean cloth with soap, clean the inside followed by the outside. Rinse with water and remove excess water. For goggles and face shields, keep the straps and foam from getting wet. Allow eye protection to dry or dry with a clean absorbent cloth. -

Adventuring with Books: a Booklist for Pre-K-Grade 6. the NCTE Booklist
DOCUMENT RESUME ED 311 453 CS 212 097 AUTHOR Jett-Simpson, Mary, Ed. TITLE Adventuring with Books: A Booklist for Pre-K-Grade 6. Ninth Edition. The NCTE Booklist Series. INSTITUTION National Council of Teachers of English, Urbana, Ill. REPORT NO ISBN-0-8141-0078-3 PUB DATE 89 NOTE 570p.; Prepared by the Committee on the Elementary School Booklist of the National Council of Teachers of English. For earlier edition, see ED 264 588. AVAILABLE FROMNational Council of Teachers of English, 1111 Kenyon Rd., Urbana, IL 61801 (Stock No. 00783-3020; $12.95 member, $16.50 nonmember). PUB TYPE Books (010) -- Reference Materials - Bibliographies (131) EDRS PRICE MF02/PC23 Plus Postage. DESCRIPTORS Annotated Bibliographies; Art; Athletics; Biographies; *Books; *Childress Literature; Elementary Education; Fantasy; Fiction; Nonfiction; Poetry; Preschool Education; *Reading Materials; Recreational Reading; Sciences; Social Studies IDENTIFIERS Historical Fiction; *Trade Books ABSTRACT Intended to provide teachers with a list of recently published books recommended for children, this annotated booklist cites titles of children's trade books selected for their literary and artistic quality. The annotations in the booklist include a critical statement about each book as well as a brief description of the content, and--where appropriate--information about quality and composition of illustrations. Some 1,800 titles are included in this publication; they were selected from approximately 8,000 children's books published in the United States between 1985 and 1989 and are divided into the following categories: (1) books for babies and toddlers, (2) basic concept books, (3) wordless picture books, (4) language and reading, (5) poetry. (6) classics, (7) traditional literature, (8) fantasy,(9) science fiction, (10) contemporary realistic fiction, (11) historical fiction, (12) biography, (13) social studies, (14) science and mathematics, (15) fine arts, (16) crafts and hobbies, (17) sports and games, and (18) holidays. -

Orvis False Marking Complaint 2 .Docx
Case 2:10-cv-00439-DF Document 1 Filed 10/15/10 Page 1 of 12 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS MARSHALL DIVISION Americans For Fair Patent Use, LLC § § Plaintiff, § § v. § Civil Case No. 2:10-cv-00439 § JURY The Orvis Company, Inc. § § Defendant. § § § COMPLAINT FOR FALSE PATENT MARKING Plaintiff Americans for Fair Patent Use, LLC (“AFPU”) files this complaint against Defendant The Orvis Company, Inc. (“Orvis”) and alleges as follows: NATURE OF THE ACTION 1. This is an action for false patent marking arising under 35 U.S.C. § 292. 2. As set forth in detail below, Orvis violated 35 U.S.C. § 292(a) by using false patent markings in advertising products for sale with the intent to deceive the public. 3. AFPU seeks an award of monetary damages from the Defendants, one-half of which shall be paid to the United States pursuant to 35 U.S.C. § 292(b). 4. This Court has subject matter jurisdiction over this action under 28 U.S.C. §§ 1331, 1332, 1338(a), and 1355(a). BACKGROUND 5. Orvis has violated 35 U.S.C. § 292(a) (the “False Marking Statute”) by marking unpatented articles with the intent to deceive the public. Case 2:10-cv-00439-DF Document 1 Filed 10/15/10 Page 2 of 12 6. More specifically, Orvis, with the intent to deceive the public, marked advertisements for products with a patent that is expired and, therefore, does not and cannot cover the advertised products. 7. Additionally, Orvis, with the intent to deceive the public, marked advertisements for products with the number of a patent that, even if not expired, would not cover the advertised products.