Marburg Virus Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HIV/AIDS Treatment and Care in a Long-Term Conflict Setting: Observations from the AIDS Support Organization (TASO) in the Teso Region Emma Smith SIT Study Abroad
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Spring 2008 HIV/AIDS Treatment and Care in a Long-Term Conflict Setting: Observations From The AIDS Support Organization (TASO) in the Teso Region Emma Smith SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Recommended Citation Smith, Emma, "HIV/AIDS Treatment and Care in a Long-Term Conflict Setting: Observations From The AIDS Support Organization (TASO) in the Teso Region" (2008). Independent Study Project (ISP) Collection. 99. https://digitalcollections.sit.edu/isp_collection/99 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. HIV/AIDS Treatment and Care in a Long-Term Conflict Setting: Observations from The AIDS Support Organization (TASO) in the Teso Region Emma Smith Advisor: Alutia Samuel Academic Directors: Charlotte Mafumbo and Martha Wandera Location: TASO Soroti SIT Uganda Spring 2008 Dedication To all the people living with HIV/AIDS in Teso, who continue to live strongly despite decades of suffering from continuous war, displacement and neglect. May the world come to recognize the struggles that you live with. Acknowledgements There are so many people to whom thanks is owed, it would not be possible to acknowledge them all even if time and space allowed. Primarily, I would like to thank the clients of TASO Soroti, who so willingly welcomed a stranger into their communities and allowed so many questions to be asked of them. -

BETTER GROWTH, BETTER CITIES Achieving Uganda’S Development Ambition
BETTER GROWTH, BETTER CITIES Achieving Uganda’s Development Ambition A paper by the Government of Uganda and the New Climate Economy Partnership November 2016 THE REPUBLIC OF UGANDA THE REPUBLIC OF UGANDA About this paper The analysis in this paper was produced for the New Climate Partnership in Uganda research project, culminating in the report, Achieving Uganda’s Development Ambition: The Economic Impact of Green Growth – An Agenda for Action. This National Urban Transition paper is published as a supporting working paper and provides a fuller elaboration of the urbanisation elements in the broader report. Partners Achieving Uganda’s Development Ambition: The Economic Impact of Green Growth – An Agenda for Action was jointly prepared by the Government of Uganda through the Ministry of Finance, Planning and Economic Development (MFPED), the Ugandan Economic Policy Research Centre (EPRC) Uganda, the Global Green Growth Institute (GGGI), the New Climate Economy (NCE), and the Coalition for Urban Transitions (an NCE Special Initiative). Ministry of Finance, Planning and Economic Development Plot 2/12 Apollo Kaggwa Road P.O.Box 8147 Kampala, Uganda +256-414-707000 COALITION FOR URBAN TRANSITIONS A New Climate Economy Special Initiative Acknowledgements The project team members were Russell Bishop, Nick Godfrey, Annie Lefebure, Filippo Rodriguez and Rachel Waddell (NCE); Madina Guloba (EPRC); Maris Wanyera, Albert Musisi and Andrew Masaba (MPFED); and Samson Akankiza, Jahan-zeb Chowdhury, Peter Okubal and John Walugembe (GGGI). The technical -

A Monthly Newsletter on Food Security and Vulnerability in Uganda
A Monthly Newsletter on Food Security and Vulnerability in Uganda Number 02/2001 15 February, 2001 Summary The NGO, Concern Worldwide, reports that households in Katakwi District (eastern Uganda) are experiencing moderate food insecurity. The most affected people are located in Kapelebyong and Usuk Counties on the border with Moroto District, where there is limited access to food and malnutrition rates are high. Only households in Magoro Sub-County of Usuk, received about 100 kg of maize and beans from another NGO, Hands on Service, in early December. No further assistance has been provided and there are no immediate plans to provide additional food aid to the affected people in the district. Because of the expected increase in demand on meager food supplies and other resources, many residents are worried about the Karimojong pastoralists and their normal dry season migratory pattern into Katakwi District in search of water and pastures for their livestock. Large livestock herds may cause destruction of crops and vegetation, increasing vulnerability to food insecurity and competition for already diminishing pastures. District authorities and local residents also are concerned about the possibility of increasing tensions and civil insecurity due to the presence of armed pastoralists. To ensure peace and security in the district, the Government has increased deployment of Uganda People’s Defence Force personnel. Concern Worldwide affirms that even though the population may require assistance after February 2001 when household food stocks run low, it is imperative for adequate civil security to be maintained to allow proper identification and targeting of the most affected households before carrying out any mitigation program for maximum benefit. -

WHO UGANDA BULLETIN February 2016 Ehealth MONTHLY BULLETIN
WHO UGANDA BULLETIN February 2016 eHEALTH MONTHLY BULLETIN Welcome to this 1st issue of the eHealth Bulletin, a production 2015 of the WHO Country Office. Disease October November December This monthly bulletin is intended to bridge the gap between the Cholera existing weekly and quarterly bulletins; focus on a one or two disease/event that featured prominently in a given month; pro- Typhoid fever mote data utilization and information sharing. Malaria This issue focuses on cholera, typhoid and malaria during the Source: Health Facility Outpatient Monthly Reports, Month of December 2015. Completeness of monthly reporting DHIS2, MoH for December 2015 was above 90% across all the four regions. Typhoid fever Distribution of Typhoid Fever During the month of December 2015, typhoid cases were reported by nearly all districts. Central region reported the highest number, with Kampala, Wakiso, Mubende and Luweero contributing to the bulk of these numbers. In the north, high numbers were reported by Gulu, Arua and Koti- do. Cholera Outbreaks of cholera were also reported by several districts, across the country. 1 Visit our website www.whouganda.org and follow us on World Health Organization, Uganda @WHOUganda WHO UGANDA eHEALTH BULLETIN February 2016 Typhoid District Cholera Kisoro District 12 Fever Kitgum District 4 169 Abim District 43 Koboko District 26 Adjumani District 5 Kole District Agago District 26 85 Kotido District 347 Alebtong District 1 Kumi District 6 502 Amolatar District 58 Kween District 45 Amudat District 11 Kyankwanzi District -
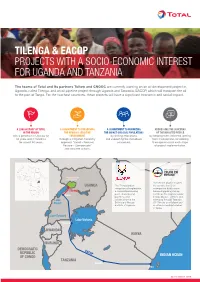
Tilenga & Eacop Projects with a Socio-Economic Interest for Uganda
TILENGA & EACOP PROJECTS WITH A SOCIO-ECONOMIC INTEREST FOR UGANDA AND TANZANIA The teams of Total and its partners Tullow and CNOOC are currently working on an oil development project in Uganda, called Tilenga, and an oil pipeline project through Uganda and Tanzania, EACOP, which will transport the oil to the port of Tanga. For the two host countries, these projects will have a significant economic and social impact. A LONG HISTORY OF TOTAL A COMMITMENT TO PRESERVING A COMMITMENT TO MINIMIZING ADDRESSING THE CONCERNS IN THE REGION THE REGION'S SENSITIVE THE IMPACT ON LOCAL POPULATIONS OF THE IMPACTED PEOPLE with a presence in Uganda for ENVIRONMENT by limiting relocations by keeping them informed, getting 60 years and in Tanzania through a mitigation hierarchy and supporting the individuals them involved and considering for almost 50 years. approach “Avoid – Reduce/ concerned. their opinions into each stage Restore – Compensate” of project implementation. and concrete actions. SOUDAN DU SUD ÉTHIOPIE Murchison Falls National Park The EACOP project involves UGANDA The Tilenga project the construction of an comprises oil exploration, underground hydrocarbon Tilenga a crude oil processing transport pipeline starting Hoima plant, underground just inside the Uganda border pipelines, and (Hoima District - 297km) and Lake infrastructure in the extending through Tanzania Albert Buliisa and Nwoya (1147km) to an oil depot and districts of Uganda. an offshore loading terminal in Tanga. Lake Edward Lake Victoria Bukoba RWANDA KENYA BURUNDI DEMOCRATIC EACOP REPUBLIC INDIAN OCEAN OF CONGO TANZANIA Singida Tanga SEPTEMBER 2019 ZAMBIE MOZAMBIQUE MALAWI SOUDAN DU SUD THIOPIE FOCUS ON THE TILENGA PROJECT Total E&P Uganda, fully aware of the project's sensitive nature, has placed particular emphasis on environmental and societal issues, with a specific commitment to leaving the site in a better state than it was before the work started and to limiting residents' relocations as much as possible. -

Kalungu District
National Population and Housing Census 2014 Area Specific Profiles Kalungu District April 2017 National Population and Housing Census 2014 Area Specific Profiles – Kalungu District This report presents findings of National Population and Housing Census (NPHC) 2014 undertaken by the Uganda Bureau of Statistics (UBOS). Additional information about the Census may be obtained from the UBOS Head Office, Statistics House. Plot 9 Colville Street, P. O. Box 7186, Kampala, Uganda; Telephone: +256-414 706000 Fax: +256-414 237553; E-mail: [email protected]; Website: www.ubos.org Cover Photos: Uganda Bureau of Statistics Recommended Citation Uganda Bureau of Statistics 2017, The National Population and Housing Census 2014 – Area Specific Profile Series, Kampala, Uganda. National Population and Housing Census 2014 Area Specific Profiles – Kalungu District FOREWORD Demographic and socio-economic data are useful for planning and evidence-based decision making in any country. Such data are collected through Population Censuses, Demographic and Socio-economic Surveys, Civil Registration Systems and other Administrative sources. In Uganda, however, the Population and Housing Census remains the main source of demographic data, especially at the sub-national level. Population Census taking in Uganda dates back to 1911 and since then the country has undertaken five such Censuses. The most recent, the National Population and Housing Census 2014, was undertaken under the theme ‘Counting for Planning and Improved Service Delivery’. The enumeration for the 2014 Census was conducted in August/September 2014. The Uganda Bureau of Statistics (UBOS) worked closely with different Government Ministries, Departments and Agencies (MDAs) as well as Local Governments (LGs) to undertake the census exercise. -

RCDF PROJECTS in KABAROLE DISTRICT, UGANDA UCC Support
Rural Communications Development Fund (RCDF) RCDF PROJECTS IN KABAROLE DISTRICT, UGANDA MAP O F KABAR O LE D ISTR IC T SHO W IN G SU B C O U N TIES N Hakiba ale Kicwa mba Western Buk uk u Busoro Karam bi Ea ste rn Mugu su So uthe rn Buh ees i Kisom oro Rutee te Kibiito Rwiimi 10 0 10 20 Km s UCC Support through the RCDF Programme Uganda Communications Commission Plot 42 -44, Spring road, Bugolobi P.O. Box 7376 Kampala, Uganda Tel: + 256 414 339000/ 312 339000 Fax: + 256 414 348832 E-mail: [email protected] Website: www.ucc.co.ug 1 Table of Contents 1- Foreword……………………………………………………………….……….………..…..…....….…3 2- Background…………………………………….………………………..…………..….….……………4 3- Introduction………………….……………………………………..…….…………….….…….……..4 4- Project profiles……………………………………………………………………….…..…….……...5 5- Stakeholders’ responsibilities………………………………………………….….…........…12 6- Contacts………………..…………………………………………….…………………..…….……….13 List of tables and maps 1- Table showing number of RCDF projects in Kabarole district………….…….….5 2- Map of Uganda showing Kabarole district………..………………….………...….….14 10- Map of Kabarole district showing sub counties………..…………………………..15 11- Table showing the population of Kabarole district by sub counties……….15 12- List of RCDF Projects in Kabarole district…………………………………….…….….16 Abbreviations/Acronyms UCC Uganda Communications Commission RCDF Rural Communications Development Fund USF Universal Service Fund MCT Multipurpose Community Tele-centre PPDA Public Procurement and Disposal Act of 2003 POP Internet Points of Presence ICT Information and Communications Technology UA Universal Access MoES Ministry of Education and Sports MoH Ministry of Health DHO District Health Officer CAO Chief Administrative Officer RDC Resident District Commissioner 2 1. Foreword ICTs are a key factor for socio-economic development. -

Maternal Healthcare in Eastern Uganda: the Three Delays, Mothers Making Empowered Choices, and Combatting Maternal Mortality Emma Gier SIT Study Abroad
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Summer 2016 Maternal Healthcare in Eastern Uganda: The Three Delays, Mothers Making Empowered Choices, and Combatting Maternal Mortality Emma Gier SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the African Studies Commons, Family Medicine Commons, Health Policy Commons, Maternal and Child Health Commons, Nursing Midwifery Commons, Obstetrics and Gynecology Commons, Pediatrics Commons, and the Women's Health Commons Recommended Citation Gier, Emma, "Maternal Healthcare in Eastern Uganda: The Three Delays, Mothers Making Empowered Choices, and Combatting Maternal Mortality" (2016). Independent Study Project (ISP) Collection. 2442. https://digitalcollections.sit.edu/isp_collection/2442 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Fall 16 Maternal Healthcare in Eastern Uganda: The Three Delays, Mothers Making Empowered Choices, and Combatting Maternal Mortality Emma Gier Charlotte Mafumbo | SIT Uganda: Development Studies Fall 2016 Eastern Uganda: Mbale District, Manafwa District and Kween District “She’s happy. She comes and she smiles with her beautiful baby girl. So, you touch people’s lives and likewise their lives touch you sometimes. It’s really nice being w ith people.” – A M i d w i f e I want to dedicate this project to all mothers, as being a mother is the most difficult job around. -

Funding Going To
% Funding going to Funding Country Name KP‐led Timeline Partner Name Sub‐awardees SNU1 PSNU MER Structural Interventions Allocated Organizations HTS_TST Quarterly stigma & discrimination HTS_TST_NEG meetings; free mental services to HTS_TST_POS KP clients; access to legal services PrEP_CURR for KP PLHIV PrEP_ELIGIBLE Centro de Orientacion e PrEP_NEW Dominican Republic $ 1,000,000.00 88.4% MOSCTHA, Esperanza y Caridad, MODEMU Region 0 Distrito Nacional Investigacion Integral (COIN) PrEP_SCREEN TX_CURR TX_NEW TX_PVLS (D) TX_PVLS (N) TX_RTT Gonaives HTS_TST KP sensitization focusing on Artibonite Saint‐Marc HTS_TST_NEG stigma & discrimination, Nord Cap‐Haitien HTS_TST_POS understanding sexual orientation Croix‐des‐Bouquets KP_PREV & gender identity, and building Leogane PrEP_CURR clinical providers' competency to PrEP_CURR_VERIFY serve KP FY19Q4‐ KOURAJ, ACESH, AJCCDS, ANAPFEH, APLCH, CHAAPES, PrEP_ELIGIBLE Haiti $ 1,000,000.00 83.2% FOSREF FY21Q2 HERITAGE, ORAH, UPLCDS PrEP_NEW Ouest PrEP_NEW_VERIFY Port‐au‐Prince PrEP_SCREEN TX_CURR TX_CURR_VERIFY TX_NEW TX_NEW_VERIFY Bomu Hospital Affiliated Sites Mombasa County Mombasa County not specified HTS_TST Kitui County Kitui County HTS_TST_NEG CHS Naishi Machakos County Machakos County HTS_TST_POS Makueni County Makueni County KP_PREV CHS Tegemeza Plus Muranga County Muranga County PrEP_CURR EGPAF Timiza Homa Bay County Homa Bay County PrEP_CURR_VERIFY Embu County Embu County PrEP_ELIGIBLE Kirinyaga County Kirinyaga County HWWK Nairobi Eastern PrEP_NEW Tharaka Nithi County Tharaka Nithi County -

RCDF PROJECTS in NTUNGAMO DISTRICT, UGANDA UCC Support
Rural Communications Development Fund (RCDF) RCDF PROJECTS IN NTUNGAMO DISTRICT, UGANDA MAP O F N TU N G AM O D ISTR IC T SH O W IN G SU B C O U N TIES N K ib atsi Ito jo B won gye ra Ih un ga Ntun ga mo TC Nyakyera Nya biho ko Ru ko ni Ntu ng amo Ru ga ram a Ruh aa ma Ru ba are Rw eikiniro Ng om a Kay on z a 10 0 10 20 Km s UCC Support through the RCDF Programme Uganda Communications Commission Plot 42 -44, Spring road, Bugolobi P.O. Box 7376 Kampala, Uganda Tel: + 256 414 339000/ 312 339000 Fax: + 256 414 348832 E-mail: [email protected] Website: www.ucc.co.ug 1 Table of Contents 1- Foreword……………………………………………………………….……….………..…..…....….…3 2- Background…………………………………….………………………..…………..….….……………4 3- Introduction………………….……………………………………..…….…………….….……….…..4 4- Project profiles……………………………………………………………………….…..…….……...5 5- Stakeholders’ responsibilities………………………………………………….….…........…12 6- Contacts………………..…………………………………………….…………………..…….……….13 List of tables and maps 1- Table showing number of RCDF projects in Ntungamo district………….……….5 2- Map of Uganda showing Ntungamo district………..………………….………...…….14 10- Map of Ntungamo district showing sub counties………..………………………….15 11- Table showing the population of Ntungamo district by sub counties……...15 12- List of RCDF Projects in Ntungamo district…………………………………….…….…16 Abbreviations/Acronyms UCC Uganda Communications Commission RCDF Rural Communications Development Fund USF Universal Service Fund MCT Multipurpose Community Tele-centre PPDA Public Procurement and Disposal Act of 2003 POP Internet Points of Presence ICT Information and Communications Technology UA Universal Access MoES Ministry of Education and Sports MoH Ministry of Health DHO District Health Officer 2 CAO Chief Administrative Officer RDC Resident District Commissioner 1. -

Pastoralism in Karamoja
Pastoralism in Karamoja Assessment of factors affecting pastoralist lifestyles in Moroto, Amudat and Kaabong May 2016 1 1 Photos taken in Kaabong and Moroto by Sumy Sadurni, for ACTED TABLE OF CONTENTS Table of Contents ................................................................................................................................... 2 Acknowledgements ................................................................................................................................ 3 Executive Summary ................................................................................................................................ 4 Background ............................................................................................................................................ 4 Methodology .......................................................................................................................................... 5 Main findings ......................................................................................................................................... 7 Changes to Pastoralist Livestyle ............................................................................................................................................................ 7 Agriculture .......................................................................................................................................................................................................... 7 Conflict ................................................................................................................................................................................................................. -

BUKWO BFP.Pdf
Local Government Budget Framework Paper Vote: 567 Bukwo District Structure of Budget Framework Paper Foreword Executive Summary A: Revenue Performance and Plans B: Summary of Department Performance and Plans by Workplan C: Draft Annual Workplan Outputs for 2014/15 Page 1 Local Government Budget Framework Paper Vote: 567 Bukwo District Foreword Bukwo District Local Government Council appreciates the importance of preparing Budget Framework Paper (BFP) not only as a requirement in the guidelines Governing Local Governments planning process but as a necessary document in guiding the development partners and all other Pertinent stakeholders in improvement of service delivery to people of Bukwo district. This BFP takes into consideration the priorities of the people of Bukwo district that have been obtained through participatory planning which leads to accomplishment of the District Goal and therefore Vision. It has been formulated taking into account the budget ceiling by Local government finance Commission, expected Donor funding and projected Local revenue as well as cross-cutting issues of gender, environment, HIV/AIDS, employment, population, social protection and income distribution. We also appreciate the development partners for contributing direct monetary support of UGX. 448 million (i.e. Strengthening Decentralisation for Sustainability (SDS), WHO/UNICEF, UNFPA, Global Fund will contribute respectively 250million, 70 million, 27 million and global fund 100million ) and Off-budget support of UGX. 823 million (I.e. SDS, SUNRISE OVC, STAR-E, SURE and Marie stopes contributes respectively 312million, 17 million 250 million, 70 million and 195 million). I therefore take this opportunity to thank all the pertinent stakeholders who contributed in the preparation of this Budget Framework Paper.