The NEURONS and NEURAL SYSTEM: a 21St CENTURY PARADIGM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Designing Biological Systems
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Designing biological systems David A. Drubin,1 Jeffrey C. Way,2 and Pamela A. Silver1,3 1Department of Systems Biology, Harvard Medical School, Boston, Massachusetts 02115, USA; 2EMD Lexigen Research Center, Billerica, Massachusetts 01821, USA The design of artificial biological systems and the under- chemical systems that mimic living systems, especially standing of their natural counterparts are key objectives in regard to replication and Darwinian selection. This of the emerging discipline of synthetic biology. Toward review will primarily focus on accomplishments of the both ends, research in synthetic biology has primarily first strategy; however, readers are referred to several fine focused on the construction of simple devices, such as reviews on the generation of artificial, life-emulating transcription-based oscillators and switches. Construc- systems (Szostak et al. 2001; Benner 2003; Benner and tion of such devices should provide us with insight on Sismour 2005). the design of natural systems, indicating whether our According to the synthetic biology paradigm, a syn- understanding is complete or whether there are still gaps thetically programmed cell should be composed of many in our knowledge. Construction of simple biological sys- subsystems that operate successfully as a result of ex- tems may also lay the groundwork for the construction tensive characterization and educated design. System of more complex systems that have practical utility. To construction is the product of iterative cycles of com- realize its full potential, biological systems design bor- puter modeling, biological assembly, and testing. In this rows from the allied fields of protein design and meta- way, much from the field of systems biology can be ap- bolic engineering. -

The Device Approach to Emergent Properties
arXiv:1801.05452, Version 3 Asking Biological Questions of Physical Systems: the Device Approach to Emergent Properties Bob Eisenberg Department of Applied Mathematics Illinois Institute of Technology USA Department of Physiology and Biophysics Rush University USA [email protected] January 17, 2018 9/23/2021 9:14 AM Abstract Life occurs in concentrated ‘Ringer Solutions’ derived from seawater that Lesser Blum studied for most of his life. As we worked together, Lesser and I realized that the questions asked of those solutions were quite different in biology from those in the physical chemistry he knew. Biology is inherited. Information is passed by handfuls of atoms in the genetic code. A few atoms in the proteins built from the code change macroscopic function. Indeed, a few atoms often control biological function in the same sense that a gas pedal controls the speed of a car. Biological questions then are most productive when they are asked in the context of evolution. What function does a system perform? How is the system built to perform that function? What forces are used to perform that function? How are the modules that perform functions connected to make the machinery of life. Physiologists have shown that much of life is a nested hierarchy of devices, one on top of another, linking atomic ions in concentrated solutions to current flow through proteins, current flow to voltage signals, voltage signals to changes in current flow, all connected to make a regenerative system that allows electrical action potentials to move meters, under the control of a few atoms. -
S I Section 4
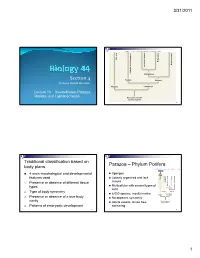
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Systems Biology and Its Relevance to Alcohol Research
Commentary: Systems Biology and Its Relevance to Alcohol Research Q. Max Guo, Ph.D., and Sam Zakhari, Ph.D. Systems biology, a new scientific discipline, aims to study the behavior of a biological organization or process in order to understand the function of a dynamic system. This commentary will put into perspective topics discussed in this issue of Alcohol Research & Health, provide insight into why alcohol-induced disorders exemplify the kinds of conditions for which a systems biological approach would be fruitful, and discuss the opportunities and challenges facing alcohol researchers. KEY WORDS: Alcohol-induced disorders; alcohol research; biomedical research; systems biology; biological systems; mathematical modeling; genomics; epigenomics; transcriptomics; metabolomics; proteomics ntil recently, most biologists’ emerging discipline that deals with, Alcohol Research & Health intend to efforts have been devoted to and takes advantage of, these enormous address. In this commentary, we will Ureducing complex biological amounts of data. Although scientists try to put the topics discussed in this systems to the properties of individual and engineers have applied the concept issue into perspective, provide views molecules. However, with the com of an integrated systemic approach for on the significance of systems biology pleted sequencing of the genomes of years, systems biology has only emerged as a new, distinct discipline to study 1 High-throughput genomics is the study of the structure humans, mice, rats, and many other and function of an organism’s complete genetic content, organisms, technological advances in complex biological systems in the past or genome, using technology that analyzes a large num the fields of high-throughput genomics1 several years. -

Communication Theory in Biological Systems
SUMMARY CDI TYPE I: A Communications Theory Approach to Morphogenesis and Architecture Maintenance The assertion that biological systems are communication networks would draw no rebuke from biologists – the terms signaling, communication, and network are deeply embedded parts of the biology parlance. However, the more profound meanings of information and communication are often overlooked when considering biological systems. Information can be quantified, its flow can be measured and tight bounds exist for its representation and conveyance between transmitters and receivers in a variety of settings. Furthermore, communications theory is about efficient com- munication where energy is at a premium – as is often the case in organisms. But perhaps most important, information theory allows mechanism-blind bounds on decisions and information flow. That is, the physics of a system allows determination of limits that any method of information description, delivery or processing must obey. Thus, rigorous application of communication theory to complex multi-cellular biological sys- tems seems both attractive and obvious as an organizing principle – a way to tease order from the myriad engineering solutions that comprise biological systems. Likewise, study of biological systems – engineering solutions evolved over eons – might yield new communication and com- putation theory. Yet so far, a communications-theoretic approach to multi-cellular biology has received scant, if any, attention. We therefore propose to explore this interdisciplinary intellectual -

Animal Diversity Part 2
Textbook resources • pp. 517-522 • pp. 527-8 Animal Diversity • p. 530 part 2 • pp. 531-2 Clicker question In protostomes A. The blastopore becomes the mouth. B. The blastopore becomes the anus. C. Development involves indeterminate cleavage. D. B and C Fig. 25.2 Phylogeny to know (1). Symmetry Critical innovations to insert: Oral bilateral symmetry ecdysis mouth develops after anus multicellularity Aboral tissues 1 Animal diversity, part 2 Parazoa Diversity 2 I. Parazoa • Porifera: Sponges II. Cnidaria & Ctenophora • Tissues • Symmetry I. Outline the • Germ Layers III. Lophotrochozoa unique • Embryonic characteristics Development of sponges IV. Ecdysozoa • Body Cavities • Segmentation Parazoa Parazoa • Porifera: Sponges • Porifera: Sponges – Multicellular without – Hermaphrodites tissues – Sexual and asexual reproduction – Choanocytes (collar cells) use flagella to move water and nutrients into pores – Intracellular digestion Fig. 25.11 Animal diversity, part 2 Clicker Question Diversity 2 I. Parazoa In diploblastic animals, the inner lining of the digestive cavity or tract is derived from II. Cnidaria & Ctenophora A. Endoderm. II. Outline the B. Ectoderm. unique III. Lophotrochozoa C. Mesoderm. characteristics D. Coelom. of cnidarians and IV. Ecdysozoa ctenophores 2 Coral Box jelly Cnidaria and Ctenophora • Cnidarians – Coral; sea anemone; jellyfish; hydra; box jellies • Ctenophores – Comb jellies Sea anemone Jellyfish Hydra Comb jelly Cnidaria and Ctenophora Fig. 25.12 Coral Box jelly Cnidaria and Ctenophora • Tissues Fig. 25.12 – -

BIOL 2290 - 3 EVOLUTION of ANIMAL BODY PLANS (3,0,3) Winter 2020
BIOL 2290 - 3 EVOLUTION OF ANIMAL BODY PLANS (3,0,3) Winter 2020 Instructor: Dr. Louis Gosselin Office: Research Centre RC203 Email: [email protected] Course website: www.faculty.tru.ca/lgosselin/biol2290/ Meeting times Lectures: Mon 8:30 - 9:45 [AE 162] Wed 8:30 - 9:45 [AE 162] Laboratories - 3 hours per week [S 378] Calendar description This course explores the spectacular diversity of animal body plans, and examines the sequence of events that lead to this diversity. Lectures and laboratories emphasize the inherent link between body form, function and phylogeny. The course also highlights the diverse roles that animals play in natural ecosystems as well as their implications for humans, and examines how knowledge of animal morphology, development, and molecular biology allows us to reconstruct the phylogenetic tree of the Animalia. Educational objectives Upon successful completion of this course, students will be able to identify the major evolutionary events that lead to the current diversity of animal body plans, and the selective pressures that favoured each type of body plan. They will be able to recognize the functional significance of animal body design, relating morphology with specific lifestyles. Students will be able to describe the major functions that animals play in natural ecosystems and the significance of different animals for humans. In addition, students successfully completing the laboratory component of the course will be skilled in dissection techniques, identification, and in the basic design, execution and analysis of a scientific experiment with animals. Prerequisites BIOL1110, BIOL1210 Required texts Ruppert EE, Fox RS, Barnes RD (2004) Invertebrate Zoology. -

FISH310: Biology of Shellfishes
FISH310: Biology of Shellfishes Lecture Slides #3 Phylogeny and Taxonomy sorting organisms How do we classify animals? Taxonomy: naming Systematics: working out relationships among organisms Classification • All classification schemes are, in part, artificial to impose order (need to start some where using some information) – Cell number: • Acellular, One cell (_________), or More than one cell (metazoa) – Metazoa: multicellular, usu 2N, develop from blastula – Body Symmetry – Developmental Pattern (Embryology) – Evolutionary Relationship Animal Kingdom Eumetazoa: true animals Corals Anemones Parazoa: no tissues Body Symmetry • Radial symmetry • Phyla Cnidaria and Ctenophora • Known as Radiata • Any cut through center ! 2 ~ “mirror” pieces • Bilateral symmetry • Other phyla • Bilateria • Cut longitudinally to achieve mirror halves • Dorsal and ventral sides • Anterior and posterior ends • Cephalization and central nervous system • Left and right sides • Asymmetry uncommon (Porifera) Form and Life Style • The symmetry of an animal generally fits its lifestyle • Sessile or planktonic organisms often have radial symmetry • Highest survival when meet the environment equally well from all sides • Actively moving animals have bilateral symmetry • Head end is usually first to encounter food, danger, and other stimuli Developmental Pattern • Metazoa divided into two groups based on number of germ layers formed during embryogenesis – differs between radiata and bilateria • Diploblastic • Triploblastic Developmental Pattern.. • Radiata are diploblastic: two germ layers • Ectoderm, becomes the outer covering and, in some phyla, the central nervous system • Endoderm lines the developing digestive tube, or archenteron, becomes the lining of the digestive tract and organs derived from it, such as the liver and lungs of vertebrates Diploblastic http://faculty.mccfl.edu/rizkf/OCE1001/Images/cnidaria1.jpg Developmental Pattern…. -

The Bioanalysis Glossary
November 2014 Bioanalysis Vol. 6 No. 16 Suppl. 1 The Bioanalysis Glossary Brought to you by PUSHING THE LIMITS IN SENSITIVITY Exceedingly sensitive. Sharply focused. THE 6500 SERIES WITH IONDRIVE™ TECHNOLOGY See what couldn’t be seen. Until now. The new AB SCIEX 6500 LC/MS/MS series with multi-component IonDrive™ technology is the world’s most sensitive triple quadrupole, improving sensitivity up to 10X and detector dynamic range by 20X over the best selling high performance triple quad – with no compromise in mass range. Unique QTRAP® linear ion trap technology and optional SelexION™ differential ion mobility technology help enhance data quality and improve throughput while reducing the need for sample preparation. When merged with the Eksigent ekspert™ microLC 200 system, the functionally stackable design reduces lab space by 100%, while minimizing maintenance costs and reducing mobile phase costs by up to 95%. The new AB SCIEX 6500 Series. It’s the farsighted successor to a long line of leading AB SCIEX mass spec systems. Explore visionary sensitivity at www.absciex.com/6500-CEN © 2012 AB SCIEX. For Research Use Only. Not for use in diagnostic procedures. The trademarks mentioned herein are the property of AB SCIEX Pte. Ltd. or their respective owners. The Bioanalysis Glossary Contents The Bioanalysis Glossary November 2014 Bioanalysis Vol. 6 No. 16 Suppl. 1 Brought to you by Bioanalysis and Bioanalysis Zone S5 FOREWORD S6 SCOPE S7 TERMS & DEFINITIONS S84 INDEX S89 COMPANY PROFILES Publishing information ISBN PDF: 978-1-910420-53-2 ISBN ePub: 978-1-910420-53-9 ISBN print: 978-1-910420-54-6 Publication history First edition published November 2014 S3 Bioanalysis (2014) 6(16) Suppl. -

The Biological Person
03-Hutchison P&E-45383.qxd 8/14/2007 6:22 PM Page 79 CHAPTER 3 THE BIOLOGICAL PERSON Stephen French Gilson Key Ideas 81 Case Study 3.1: Cheryl’s Brain Injury 82 Case Study 3.2: A Diabetes Diagnosis for Bess 82 Case Study 3.3: Melissa’s HIV Diagnosis 83 Case Study 3.4: Lifestyle Changes for Thomas 83 Case Study 3.5: Max’s Postpolio Syndrome 84 Case Study 3.6: Juan and Belinda’s Reproductive Health 84 An Integrative Approach for Understanding the Intersection of Interior Biological Health and Illness and Exterior Environment Factors 85 A Look at Six Systems 88 Nervous System 88 Endocrine System 93 Immune System 96 Cardiovascular System 100 Musculoskeletal System 103 Reproductive System 107 79 03-Hutchison P&E-45383.qxd 8/14/2007 6:22 PM Page 80 80 THE MULTIPLE DIMENSIONS OF PERSON Exterior Socioeconomic Environment/Interior Health Environment 112 Implications for Social Work Practice 114 Key Terms 115 Active Learning 115 Web Resources 115 03-Hutchison P&E-45383.qxd 8/14/2007 6:22 PM Page 81 Chapter 3 The Biological Person 81 What do social workers need to know about the interior environment of human biology? How does knowledge of biology contribute to important roles that social workers play in individual and community health and illness? KEY IDEAS As you read this chapter, take note of these central ideas: 1. Although there are a variety of ways that we can think about our bodies, the approach proposed here locates understandings of the body within theories of environments, in this case, the interior environment. -

Past, Present, and Future of Closed Human Life Support Ecosystems - a Review
47th International Conference on Environmental Systems ICES-2017-311 16-20 July 2017, Charleston, South Carolina Past, Present, and Future of Closed Human Life Support Ecosystems - A Review Christine M. Escobar1 and Dr. James A. Nabity2 University of Colorado at Boulder, Boulder, CO, 80309 During the development of human space exploration, the idea of simulating the Earth’s biosphere to provide human life support led to the convergence of space biology and the field of ecology to develop closed manmade ecological systems. A space habitat can be thought of as an ecological system of human beings exchanging energy and material within a spacecraft. In order to understand and control material and energy exchange processes, one must combine basic ecological principles with the knowledge gained through Bioregenerative Life Support Systems (BLSS) research to date. Experimentation in closed manmade ecosystems for spacecraft life support began in the 1960’s and over the years has shown biological life support systems to be realizable. By building on lessons learned, we can identify and prioritize future research objectives. Future development should focus on improved reliability of mechanical components, autonomous ecosystem control, closure of the carbon cycle (food generation and waste recycling), and the maintenance of long-term stability and robustness. A common need identified throughout all closed ecological life support systems (CELSS) research is mass and energy exchange models that enable intelligent autonomous control and design optimization. To validate mass balance models, integrated system level experiments are needed, but full-scale tests are time consuming and expensive. Small closed experimental ecosystems (microcosms) could allow observation of stability limits and response to perturbation with repeated short duration experiments. -

Biological Effectiveness of Neutrons
BIOLOGICAL EFFECTIVENESS OF NEUTRONS Research Needs February 1994 Submitted to The Science Panel of the Committee on Interagency Radiation Research and Policy Coordination Prepared by G.W. Casarett, L.A. Braby, J.J. Broerse, MM. Elkind, D.T, Goodhead, and N.L. Oleinick ER CNSTFHBUTiON OF THIS DOCUMENT IS UNUMfTED DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, make any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. COMMITTEE ON INTERAGENCY RADIATION RESEARCH AND POLICY COORDINATION 1019 Nineteenth Street, NW, Suite 700 Washington, D.C 20036 May 31, 1994 Dr. Alvin L. Young Chairman, CIRRPC Office of Agricultural Biotechnology U.S. Department of Agriculture 14th and Independence Avenue, S.W. Washington, DC 20250-2200 Dear Dr. Young: As Chairman of the Science Panel of the Committee on Interagency Radiation Research and Policy Coordination (CIRRPC), I am pleased to provide you with the final report on the Biological Effectiveness of Neutrons: Research Needs.