The Modulation and Organisation of State-Dependent Brain Networks in Attention-Deficit/Hyperactivity Disorder
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rest Lawn Memorial Park Burial Book 1
REST LAWN MEMORIAL PARK BURIAL BOOK Surname FIRST NAME BIRTH-DATE DEATH or GARD SEC BLK PLOT BURIAL DATE EN Abbey Gaither William 1939 25-Mar-1963 BET 273 A 1 Abbey Geneva Hope 10-Oct-1919 18-Oct-1942 BET 273 A 2 Ackerby Seth 08/27/1878 30-Mar-1953 HOP 14 2 Adams Bertha V. 11-Jan-1909 13-Feb-2001 ARB 136 B 10 Adams Carsen 24-Dec-1918 30-Oct-1971 BET 229 B 9 Adams Eleanor 2-Nov-1937 15-Dec-2000 BET 216 B 3 Adams Elmer C. 09/18/1888 30-Aug-1981 CED 364 3 Adams Elmer Floyd 27-Jan-1917 20-Jul-1960 CED 364 6 Adams Gary Pearl 28-Apr-1941 23-Oct-1996 BET 229 B 8 Adams James Victor 12-Mar-1901 7-Mar-1974 ARB 136 B 9 Adams Lucille 4-Dec-1920 12-Jun-2013 BET 229 B 10 Adams Mabel A. 09/14/1888 28-Nov-1984 CED 364 4 Adams Marion A. 21-Apr-1928 14-Aug-2000 ARB 136 B 7 Agee Della E. 1874 15-Apr-1951 BET 249 A 2 Agee James R. 1868 15-Jul-1936 BET 249 A 1 Aho Beatrice Thompson 18-Jun-1914 28-May-1999 BET 185 B 2 Aho Toivo 9-Mar-1908 27-Feb-1983 BET 186 B 2 Akers Bertha M. 16-May-1909 9-Oct-1910 ARB 30 0 1 Akers Izorab 02/07/1863 05/08/1889 ARB 68 0 5 Akers Jabez H. 04/15/1827 30-Sep-1909 ARB 68 0 4 Akers Mabel Hoel 1879 28-Feb-1962 ARB 30 0 3 Akers Medley E. -

8Th International Conference on Isotopes and Expo
th 2 0 1 4 8th International Conference on Isotopes and Expo Preparing for Tomorrow Sponsored by the Accelerator Applications, Preliminary Program Biology & Medicine, and Isotope & Radiation Divisions of the American Nuclear Society www.8ici.org August 24-28, 2014 Hyatt Regency-Chicago Chicago, IL SPONSORS Accelerator Applications Division Biology and Medicine Division Isotopes and Radiation Division 2 2014 International Conference on Isotopes and Expo: Preliminary Program www.ans.org Table of Contents Plenary Programs and Speakers Sponsors 2 Meeting Officials 4-5 Meeting Information and Special Events 6 Plenary Programs and Speakers 7 Meeting Schedule 8-9 Monday Technical Sessions 10-13 Tuesday Technical Sessions 14-18 Wednesday Technical Sessions 19-23 Thursday Technical Sessions 23-24 2014 8TH ICI Registration Form 25 www.8ici.org 2014 International Conference on Isotopes and Expo: Preliminary Program 3 Meeting Officials Honorary Chair: General Chair: Assistant General Chair: Myung-Chul Lee Paul T. Dickman Nigel R. Stevenson President, WCI Argonne National Laboratory Clear Vascular, Inc. President, Korean Association for Radiation Application Technical Program Co-Chair: Technical Program Co-Chair: Finance Chair: Rolf Zeisler Stephen P. LaMont James T. Tanner National Institute of Standard Los Alamos National Laboratory U.S. Food and Drug Administration and Technology (retired) Publications Co-Chair: Publications Co-Chair: International Program Director: Sam Glover Bryan P. Bednarz Gulbarshyn Bozheyeva University of Cincinnati University of Wisconsin-Madison MELE Associates, Inc. Executive Advisory Board Executive Advisory Board: Executive Advisory Board: JongKyung Kim Meera Venkatesh Ron Cameron WCI, Secretary-General IAEA OECD-NEA President, KAERI Executive Advisory Board Member: President Elect, WCI International Coordinator WCI: Ilham Y. -

2006 Annual Report Board of Directors Kathryn R
Legal Aid Society of Middle Tennessee and the Cumberlands 2006 Annual Report Board of Directors Kathryn R. Edge, President N. Houston Parks, First Vice President Susan L. Kay, Second Vice President Richard K. Evans, Third Vice President Valerie Martin, Secretary John Andrew Goddard, Treasurer John Pellegrin, Past President Charles H. Warfield, Executive Committee Member at Large Clisby Barrow John T. Blankenship Richard M. Brooks Melanie T. Cagle Robert Allen Dickens Roberta Dobbins Trudy Edwards Daniel B. Eisenstein Craig Fickling Barbara Gooch Fannie J. Harris Amy T. Hollars G. Wilson Horde Lou Lavender Turner McCullough, Jr. James D. Petersen Teresa Poston Adrie Mae Rhodes Steve Rhodey Denice Scott Keith S. Smartt Gregory D. Smith Guilford F. Thorton, Jr. James L. Weatherly, Jr. Shelby York Nashville Pro Bono Program Board Mary Griffin, Chair Andrée Blumstein Daniel B. Eisenstein Richard Green Tonya Mitchem Grindon Susan L. Kay N. Sue Van Sant Palmer Jonathan E. Richardson Robyn L. Ryan Thor Y. Urness Mark H. Westlake Message From the President of the Board Dear Friends and Colleagues: Legal Aid Society provides a helping hand to those who need it most – low-income citizens who cannot afford professional legal help and have nowhere to turn. When they receive the help they need, when they learn that there is justice out there for everyone, then the entire community benefits. America was founded on the ideal that everyone should be treated equally under the law. And while it is still possible to find examples of justice best serving those who can afford to pay for it, Legal Aid proves that the ideals which are fundamental to a fair and just society do, indeed, exist in our nation today. -

1967 U.S. Women's Champion
1967 U.S. WOMEN'S CHAMPION Edith Lude Wear:!, lelt, pr.Hnllnq 11M cup .... hkh .hc donal~ In 1951. /9$1 U.s. Wornetn'. Champion Mrs. G/Hla Gro"er accept. lhe ClIp Im~kIfely followlno lhe toumomcml. S •• p. 190. ~ UNITED STATES ~ ._-- - - - --- -~ - ------ ---- -- -- . -- - - --~ -_. - Volume XXII Number 6 July, 1967 EDITOR: Burt Hochberg ------- --- --- --- -- CONTENTS Sarajevo 1967, by Dimitrije Bjelica .... ... ... ...... ... ...... .... ........... .............. 184 PRESIDENT Marshall Rohland Twa Games Fram Sara jevo, by Robert Byrne ... ... ......... ... ...... ................ 185 VICI·PRESIDENT Dutch Treat, by Bernard Zuckerman ............................ .... .................... 188 Isaac Kashdan REGIONAL VICE·PRESIDENTS Chess Life, Here and There, compiled by Wm. Go ichberg ......... ... 189. 203, 204, 207, 215 NEW ENCJLAND James Bolton Harold Dolldls Ell Buurdon Women's Chess, by Kothryn Slater ..... ......... ... ...... ............ .... ..... ............ 190 EASTERN Ii Obl'M LaBeU" Lewis E. Wood MIchael Raimo The College Column, by Mark L. Schwarcz ... ...... ...... ........................... 191 MID-ATLANTIC Earl Clary Steve Carruthers RObert Erk",. Observation Point, by Miro Rodojcic ... ...... .... ... ... .. ... ...... ... ... ............... 193 SOUTHERN Phlllp Lamb I-'w t H Lah.de Carroll M. Crull U. S. Open ... ..... ... ... ..... .. .. .. ........... .. ... ... .. ...................... ..... .................... 197 GREAT LAKES Donald W. Hlldlng Dr. Harvey M~ Clellan V. E. Vandenbur g Lorry Evans on Chess ... -

High-Impact Grantmaking: the Power of Collaboration
High-Impact Grantmaking: The Power of Collaboration Each year, NW Children’s Fund Board associated with increased poverty, Strengthening Fragile Families: Grants Members explore the latest research homelessness and mental health issues in this category seek to prevent child surrounding child welfare and prepare are key risk factors for child abuse and abuse and neglect in at-risk families, and for the upcoming grant-review cycle. This neglect. support healing and stability for families year, the Board invited 10 child welfare affected by domestic violence. experts to lend new research and In this grantmaking area, NWCF insights to the discussion. Several of prioritizes programs that provide In order to support families at greatest these experts also participated as counseling and therapy, foster care risk of child maltreatment, NWCF panelists at our annual fall retreat. The programming, adoption services, and prioritizes programs aimed at building result was an invigorating exchange of comprehensive support services. We will families’ “protective factors” (see ideas that confirmed NWCF’s strategic focus on comprehensive services aimed box on page two) – parent education approach, and sparked ideas for fine- at long-term benefits--programs that not and training, support groups for tuning our grant-making processes to fit only treat, but prevent victims of abuse caregivers, family centers, supportive the ever-evolving needs of fragile from growing up to become another housing programs, emergency shelters, children and families in our area. generation of abusers. counseling, and other support services. Here are highlights in our three grantmaking areas: Healing Abused Children: The goal of these grants is to help young victims of abuse and neglect heal from early childhood traumatic experiences and find permanency and stability in their lives. -

1980 Surname
Surname Given Age Date Page Maiden Note Abbett Howard 91 6-Jan D-2 Abercrombie Levi Sr. 30-Mar E-15 Able Cora Ree 73 4-Dec C-8 Acevedo Marcelina P. 68 11-Dec D-2 Acton John Wesley 85 12-May D-1 Adam Michael Sr. 88 7-Mar B-3 Adam Millee 59 3-Mar B-6 Adam Sophie (Sister Ann 66 15-Dec B-7 Madeline) Adamczyk Josephine 82 4-May D-6 Adams Francis (Sheik) 78 14-Jul C-5 Adams Ruth Carol 41 17-Oct C-5 Adams William H. 63 21-Aug B-2 Ade Eleanor Anne 63 17-Jul C-14 Adelsperger James F. 78 22-Dec C-5 Adkins Otis C. 67 24-Jun C-1 Adler Florian F. 83 10-Aug D-2 Afflek Day Malo 18-Mar B-4 Agosto Gregorio 56 3-Dec E-2 Ahlborn Rudolph C. 81 10-Sep E-1 Ahlborn Walter W. 78 20-Jul D-1 Aikman Myrtle M. 74 26-Nov D-1 Albert Joseph (Larry) 51 11-Jan B-4 Albertson Russell A. 83 27-Jul C-7 Alderden Gertrude 76 7-Jul B-4 Aleman Sadie 21-Jan B-5 Alexander Edward L. 46 5-Aug C-3 Alexander Robert W. Jr. 65 2-Jan C-9 Alexander Sonja E. 67 11-Feb C-4 Alier Audra 65 30-Nov E-11 Brown Allen Clarence F. 57 9-May B-5 Allen Norman 79 7-Aug C-4 Picture included. Allen Rabe (Ray) 95 22-Dec C-5 Allen William J. -

Annual Report
2008-09 Cortland College Foundation Annual Report 1 2008-09 Cortland College Foundation Annual Report TABLE OF CONTENTS Note: Selecting a title below will take you directly to the page. To search for a name, a class year or any other word(s), select the Edit menu and go to Find. 3 A Message From The Chair: Overcoming Tough Times Together 4 The Cortland Fund: Alumni Give in Record Numbers 5 Alumni Profile: Ethel McCloy Smiley ’31 6 Saying ‘Thank You’ with a Scholarship 7 Alumni Profile: Gerald “Jerry” Theisen ’53, M.S. ’58 8 ASC’s $980,000 Gift Builds Scholarship Endowment 9 A Heartfelt Thanks To Our Donors Chart: SUNY Cortland 2008-09 Sources of Funding Cortland College Foundation Board of Directors 10 Lifetime Giving Recognition Societies 11-13 Partners in Leadership 13 Associate Partners in Leadership 14-15 The Lofty Elm Society 15-18 Memorial Gifts 19-20 Honorary Gifts 21-22 Our Dedicated Volunteers 23-50 Alumni Gifts 24 Chart: A Summary of Gifts 25 Chart: 2009 Class Reunion Gift Campaign 51-52 Faculty, Staff and Emeriti Gifts 52-54 Friend, Foundation and Organization Gifts 55 Parent, Student and Family Gifts 56 Corporate and Matching Gifts 57-61 Gifts in Honor of Current Students 62-63 Gifts in Honor of Seniors 64 Gifts in Kind New Look for Annual Report Preserves Our Financial, Natural Resources 2 Table of Contents A Message From The Chair Overcoming Tough Times Together Brian Murphy ’83, Chair, Cortland College Foundation Although I have been a Cortland College Foundation Board Watching the festivities in the Park Center Alumni Arena, member since 2005, I had the privilege in 2008-09 of serving I was moved by the thought of your gifts to the foundation as the board chair. -

Surnames Beginning With
Card# MineName Operator Year Month Day Surname First and Middle Name Age Fatal/Nonfatal In/Outside Occupation Nationality Citizen/Alien Single/Married #Children Mine Experince Occupation Exp. Accident Cause or Remarks Fault County Mining Dist. Film# CoalType 14 Vesta No.4 Vesta Coal 1937 2 18 Babak Frank 28 nonfatal inside brakeman Slavic single thumb caught between car bumpers Washington 21st 3600 bituminous 32 Elma No.3 Baker Whitely Coal 1938 6 14 Babalanis William 18 nonfatal inside machine miner single rock broke & fell on foot heading Somerset 24th 3601 bituminous 7 Elma No.3 Baker Whitely Coal 1937 2 2 Babalonis Charles 29 nonfatal inside scraper Lithuanian citizen single foot caught under machine unloading Somerset 24th 3600 bituminous 117 Somers Pittsburgh Coal 1937 11 2 Babar Louis 35 nonfatal inside machine miner Hungarian married fall of slate in room Fayette 9th 3600 bituminous 12 Isabella Weirton Coal 1938 5 6 Babba Harry M 46 nonfatal inside married hernia lifting timber rail room Fayette 16th 3600 bituminous 33 Colonial No.3 H C Frick Coke 1939 6 24 Babchak John J 25 nonfatal inside brakeman married caught foot in clevices broke foot Fayette 9th 3601 bituminous 66 Heisley No.3 Heisley Coal 1937 9 22 Baber Louis 20 nonfatal inside machine miner Slavic single loose strand in hoist rope hit thumb Cambria 10th 3600 bituminous 91 Springfield No.1 Springfield Coal 1939 10 27 Babich Walter 57 nonfatal inside miner married caught car & roof broke hand Cambria 10th 3601 bituminous 5 Big Bend Big Bend Coal Mining 1942 9 28 Babinscak -

Surname Given Name AKA Death Date Birth Date Binder # Page # Abbott Elmer 1922 Not Given S60 165 Abel Mary L
Batavia-Related Obituaries Index (Through 2012) Surname Given Name AKA Death Date Birth Date Binder # Page # Abbott Elmer 1922 not given S60 165 Abel Mary L. 1/25/2008 10/29/1927 32 251 Abel Miles L. 12/10/2001 3/7/1909 32 251 Abell Emma (Mrs.) 1978 ca. 1904 9 1 Abercrombie Jay A. 1993 1911 14 68 Abern Terry W. 1999 not given 19 67 Abernethy M. J. (Mrs.) 1958 not given S1 19 Abernethy Naomi Ruth (Mrs. Martin J.) 1958 1865 4 85 Abhalter Donald R. 1984 1913 9 1 Abhalter Donald R. 1984 1913 11 15 Abhalter Margaret A. (Mrs. Donald R.) 1989 1914 9 1 Abhalter Margaret A. (Mrs. Donald R.) 1989 1914 13 8 Abhalter Richard "Dick" A. 2002 1941 21 2,8 Abhalter Richard A. Dick 1/5/2002 6/30/1941 32 252 Abraham Gertrude E. nee Lunt 6/9/2002 not given 32 253 Abrahamson Maurice F. 2011 Age=92 29 1, 2 Abrahamson Robert Lee "Bob" 2001 1929 20 118 Abt Peter W. 10/24/2005 9/27/1946 32 253 Aceret David H. 2011 Age=77 29 3 Acers Hale 6/18/1899 ~1893 32 207 Ackmann Edna C. 10/18/2003 ~1910 32 253 Acres John Gordon 10/12/1898 ~1804 32 185 Adam Wilhelmina 1969 not given S6 11 Adams Andrew J. 2011 1944 29 4 Adams Carole (Mrs. Richard T.) 1972 1937 7 59 Adams Don Jules 1992 1928 14 32 Adams Donald J. 2006 1928 24 173 Adams Eldon 1979 1932 9 1 Adams Elizabeth M. -
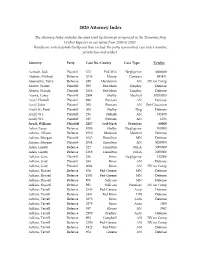
2020 Attorney Index
2020 Attorney Index The Attorney Index includes the cases tried by attorneys as reported in the Tennessee Jury Verdict Reporter in our issues from 2005 to 2020. Results are sorted alphabetically and then include the party represented, case index number, jurisdiction and verdict. Attorney Party Case No.-County Case Type Verdict Aamodt, Jodi Plaintiff 372 Fed-Win Negligence 8000000 Abelow, Michael Defense 2538 Maury Contract 995431 Abernathy, Terry Defense 240 Hardeman AN DV on Comp Abioto, Walter Plaintiff 508 Fed-Mem Employ Defense Abioto, Wanda Plaintiff 2618 Fed-Mem Employ Defense Acerra, Carey Plaintiff 2894 Shelby Medical 30035000 Acuff, Howell Plaintiff 920 Putnam AN Defense Acuff, John Plaintiff 292 Putnam AN Def-Causation Acuff, Jr., Fred Plaintiff 500 Shelby Dog Defense Acuff, W.I. Plaintiff 296 DeKalb AN 185100 Acuff, W.I. Plaintiff 285 Putnam AN 1233 Acuff, William Plaintiff 3257 Fed-Nash Premises 68000 Adair, Lacey Defense 1095 Shelby Negligence 100000 Adams, Allison Defense 2510 Madison Medical Defense Adams, Morgan Plaintiff 1625 Hamilton MN Defense Adams, Morgan Plaintiff 2068 Hamilton AN 9250000 Aden, Gareth Defense 522 Hamilton FELA 5000000 Aden, Gareth Defense 1368 Hamilton FELA 3000000 Adkins, Gary Plaintiff 216 Knox Negligence 132000 Adkins, Gary Plaintiff 838 Knox AN Defense Adkins, Gary Plaintiff 2084 Knox AN DV on Comp Adkins, Russell Defense 678 Fed-Greene MN Defense Adkins, Russell Defense 1503 Fed-Greene MN Defense Adkins, Russell Defense 976 Sullivan MN Defense Adkins, Russell Defense 981 Sullivan Premises DV -

1986 Surname
Surname Given Age Date Page Maiden Note Abegg Missel 88 15-Dec C-8 Abernathy Manuel 79 1-Jan C-5 Abner Tom 71 24-Jun B-7 Abraham Aloysius J. 77 24-Jul C-2 Abram Harold Glenn 75 14-Sep D-2 Abramson Frances L. 46 28-Dec C-7 Levine Ackerman Mary 79 16-Sep B-7 Adam Paul T. 76 10-Sep C-5 Adams Claude 78 20-Jan A-5 Adams Gloria L. 48 23-Sep D-6 Adams Irene 77 21-Aug C-3 Adams Martha 72 3-Nov C-1 Adank Gerald C. 65 1-Dec C-8 Veteran of World War II Adkins Johnnie Lee 78 13-Feb B-9 Adley Daisy A. 93 2-Sep D-7 Ahlborn Raymond W. 73 2-May C-1 Aird Gordon R. 77 21-Nov D-1 See article, p. D-1 Aitken Marion 70 1-Jul B-7 Aksentijevic Martha 56 17-Sep B-8 Alamillo Nora 75 4-Feb C-1 Albert Lester E. 65 30-Sep B-7 Albrecht Victoria A. 84 3-Jan A-7 Aldrin Raymond E. 71 11-Aug B-5 Aleksandrovic Ivan 76 11-Dec B-13 Aleksandrovic Jelena 79 2-Dec C-1 Ales Francis J. 69 31-Mar B-8 Alexander Janet 49 28-Jul C-1 Alexander Penny C. 60 24-Mar C-1 Alexander Terry 53 9-Dec C-1 Alexander Vera (Cook) 2-Dec C-1 Alexander William A. 50 27-Dec C-2 Alfaro Mark A. 21 5-Feb D-1 Alger Kenneth H. -

SURNAME A-B Name Date of Death Date of Obituary Binder Year Binder
Binder SURNAME A-B Name Date of Death Date of Obituary Binder Page Year AARON Zackery "Andy" January 6, 2013 January 9, 2013 2013 9 ABAHAZIE Duane R. May 20, 2015 June 2, 2015 2015 347 ABBADUSKA Lottie October 13, 1958 1958 37 ABBADUSKA Lottie October 13, 1958 1958 38 ABBITT Joey July 31, 1990 August 3, 1990 1990 244 ABBOTT Anna Marvene (Odell) August 13, 2008 August 14, 2008 2008 271 ABBOTT Anna Marvene (Odell) August 13, 2008 August 15, 2008 2008 272 ABBOTT Anna Marvene (Odell) August 13, 2008 August 16, 2008 2008 273 ABBOTT Bede E. November 7, 2002 November 15, 2002 2002 283 ABBOTT Boyd R. June 28, 1947 June 30, 1947 1947 89 ABBOTT Caudia July 7, 1948 July 8, 1948 1948 93 ABBOTT Charles O May 17, 1957 1957 4 ABBOTT Cleona M. February 26, 1986 February 27, 1986 1986 51A ABBOTT Cordelia May 5, 1967 1967 101 ABBOTT Dale L. September 13, 1991 September 14, 1991 1991 246 ABBOTT Donald F. May 8, 1979 May 9, 1979 1979 141 ABBOTT Donna L. August 8, 1999 August 9, 1999 1999 212 ABBOTT Dorothy September 28, 1971 1971 205 ABBOTT Dorothy M. "Dottie" September 24, 2004 September 26, 2004 2004 264 ABBOTT Elijah January 13, 1995 January 13, 1995 1995 13 ABBOTT Ella Jane December 21, 1951 December 21, 1951 1951 142 ABBOTT Ella Jane December 21, 1951 December 22, 1951 1951 142 ABBOTT Ella March 12, 1960 1960 54 ABBOTT Frances R. September 7, 1990 September 9, 1990 1990 285 ABBOTT Frances R. September 7, 1990 September 18, 1990 1990 295 ABBOTT George R.