The Origin and Abundances of the Chemical Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newsletter I
te voLUMEtd NO3 + AUTUMN 19I}9 THE NEWSLETTER I Patt of one discovery ptate of Pluto in the constellation Gemini takzn on January 29, D3A. The arow points to Plttto (about 15th magnitude). The bight star is Delta Geminorum. South is at the top and west at the left. (photo coufiesy Clyde Tombaugh) Discoverer of Pluto to Speak Dr. Hazel Ross A special treat is in store for us this Fall when Dr. Ctyde Dr. Tombaugh was born and raised on a farm, first in Tombaugh, discoverer of the planet Pluto, will give the Illinois and later in Kansas. tnspired by an uncle who was Chesley Bonestell Memorial L,ecture on September 23. As something of an amateur astronomer, and encouraged by one contemporary writer has pointed out, "More people have his father, he developed a keen interest in astronomy as a walked on the moon than have discovered planets. In fact, teenager. [,ater, using a f-inch reflector he had made only two other individuals in history have actually discovered himself, he studied Mars and Jupiter and sent drawings of new planets. Dr. Tombaugh is the third, and is still with us." what he had seen to Lowell Observatory in the hopes of obtaining a job. It turned out that the Lowetl astronomers Dr. Tombaugh had just turned 24 when, on February were looking for a young, aspiring astronomer to undertake a 18, 1930, he made the momentous discovery that rocked the search for a new planet beyond Neptune using the world. He had been working at the Lowell Observatory for observatory's new 13-inch camera. -

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Catalog of Nearby Exoplanets
Catalog of Nearby Exoplanets1 R. P. Butler2, J. T. Wright3, G. W. Marcy3,4, D. A Fischer3,4, S. S. Vogt5, C. G. Tinney6, H. R. A. Jones7, B. D. Carter8, J. A. Johnson3, C. McCarthy2,4, A. J. Penny9,10 ABSTRACT We present a catalog of nearby exoplanets. It contains the 172 known low- mass companions with orbits established through radial velocity and transit mea- surements around stars within 200 pc. We include 5 previously unpublished exo- planets orbiting the stars HD 11964, HD 66428, HD 99109, HD 107148, and HD 164922. We update orbits for 90 additional exoplanets including many whose orbits have not been revised since their announcement, and include radial ve- locity time series from the Lick, Keck, and Anglo-Australian Observatory planet searches. Both these new and previously published velocities are more precise here due to improvements in our data reduction pipeline, which we applied to archival spectra. We present a brief summary of the global properties of the known exoplanets, including their distributions of orbital semimajor axis, mini- mum mass, and orbital eccentricity. Subject headings: catalogs — stars: exoplanets — techniques: radial velocities 1Based on observations obtained at the W. M. Keck Observatory, which is operated jointly by the Uni- versity of California and the California Institute of Technology. The Keck Observatory was made possible by the generous financial support of the W. M. Keck Foundation. arXiv:astro-ph/0607493v1 21 Jul 2006 2Department of Terrestrial Magnetism, Carnegie Institute of Washington, 5241 Broad Branch Road NW, Washington, DC 20015-1305 3Department of Astronomy, 601 Campbell Hall, University of California, Berkeley, CA 94720-3411 4Department of Physics and Astronomy, San Francisco State University, San Francisco, CA 94132 5UCO/Lick Observatory, University of California, Santa Cruz, CA 95064 6Anglo-Australian Observatory, PO Box 296, Epping. -
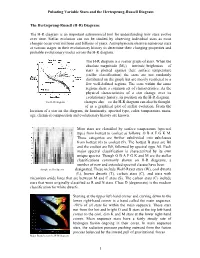
Plotting Variable Stars on the H-R Diagram Activity
Pulsating Variable Stars and the Hertzsprung-Russell Diagram The Hertzsprung-Russell (H-R) Diagram: The H-R diagram is an important astronomical tool for understanding how stars evolve over time. Stellar evolution can not be studied by observing individual stars as most changes occur over millions and billions of years. Astrophysicists observe numerous stars at various stages in their evolutionary history to determine their changing properties and probable evolutionary tracks across the H-R diagram. The H-R diagram is a scatter graph of stars. When the absolute magnitude (MV) – intrinsic brightness – of stars is plotted against their surface temperature (stellar classification) the stars are not randomly distributed on the graph but are mostly restricted to a few well-defined regions. The stars within the same regions share a common set of characteristics. As the physical characteristics of a star change over its evolutionary history, its position on the H-R diagram The H-R Diagram changes also – so the H-R diagram can also be thought of as a graphical plot of stellar evolution. From the location of a star on the diagram, its luminosity, spectral type, color, temperature, mass, age, chemical composition and evolutionary history are known. Most stars are classified by surface temperature (spectral type) from hottest to coolest as follows: O B A F G K M. These categories are further subdivided into subclasses from hottest (0) to coolest (9). The hottest B stars are B0 and the coolest are B9, followed by spectral type A0. Each major spectral classification is characterized by its own unique spectra. -

Curriculum Vitae - 24 March 2020
Dr. Eric E. Mamajek Curriculum Vitae - 24 March 2020 Jet Propulsion Laboratory Phone: (818) 354-2153 4800 Oak Grove Drive FAX: (818) 393-4950 MS 321-162 [email protected] Pasadena, CA 91109-8099 https://science.jpl.nasa.gov/people/Mamajek/ Positions 2020- Discipline Program Manager - Exoplanets, Astro. & Physics Directorate, JPL/Caltech 2016- Deputy Program Chief Scientist, NASA Exoplanet Exploration Program, JPL/Caltech 2017- Professor of Physics & Astronomy (Research), University of Rochester 2016-2017 Visiting Professor, Physics & Astronomy, University of Rochester 2016 Professor, Physics & Astronomy, University of Rochester 2013-2016 Associate Professor, Physics & Astronomy, University of Rochester 2011-2012 Associate Astronomer, NOAO, Cerro Tololo Inter-American Observatory 2008-2013 Assistant Professor, Physics & Astronomy, University of Rochester (on leave 2011-2012) 2004-2008 Clay Postdoctoral Fellow, Harvard-Smithsonian Center for Astrophysics 2000-2004 Graduate Research Assistant, University of Arizona, Astronomy 1999-2000 Graduate Teaching Assistant, University of Arizona, Astronomy 1998-1999 J. William Fulbright Fellow, Australia, ADFA/UNSW School of Physics Languages English (native), Spanish (advanced) Education 2004 Ph.D. The University of Arizona, Astronomy 2001 M.S. The University of Arizona, Astronomy 2000 M.Sc. The University of New South Wales, ADFA, Physics 1998 B.S. The Pennsylvania State University, Astronomy & Astrophysics, Physics 1993 H.S. Bethel Park High School Research Interests Formation and Evolution -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

High-Resolution Observations of the Spatial and Velocity Distribution of Cometary Hydrogen
Lunar and Planetary Institute, Houston, 1992 N 7 HIGH-RESOLUTION OBSERVATIONS OF THE SPATIAL AND VELOCITY DISTRIBUTION OF COMETARY HYDROGEN Michael E. Brown and Hyron Spinrad Dept. of Astronomy, University of California, Berkeley, CA ABSTRACT We have obtained high velocity and spatial resolution long-slit H<x spectra of comets Austin (1989cl) and Levy (1990c). Spectra of both comets clearly show the existence of a low velocity thermalized component of hydrogen gas. The amount of slow hydrogen is estimated for comet Austin. The Levy spectrum shows an unusual high-velocity spatially- confined blob of hydrogen emission of unknown origin. INTRODUCTION H<_ 6562/_ emission has been observed in comets since Kohoutek (Huppler et al. 1975, Magee-Sauer 1988). Although the excitation mechanism is weak - Ha emission is produced by fluorescence following solar Ly_ excitation of cometary hydrogen - Ho_ is observable because hydrogen is the most abundantly produced and longest-lived species in cometary comae. Hydrogen is produced primarily by photodissociation of water through the following reactions (Crovisier 1989): H20 + hv -+ H + OH. (1) OH + hv ---+H + O. (2) In reaction (1) the typical hydrogen ejection velocity is about 18 km/sec, while in reaction (2) the typical velocity is about 8 km/sec. Models of the hydrogen coma have also suggested the existence of a slow (_ 2 km/sec) thermalized component caused by hydrogen-water collision in the dense inner coma (Combi and Smyth 1987). High resolution spectroscopy of Ho_ should be able to disentangle the different velocity components of the hydrogen coma. Previous spectroscopy, using Fabry-Perot methods (Huppler et aI. -

Chapter 16 the Sun and Stars
Chapter 16 The Sun and Stars Stargazing is an awe-inspiring way to enjoy the night sky, but humans can learn only so much about stars from our position on Earth. The Hubble Space Telescope is a school-bus-size telescope that orbits Earth every 97 minutes at an altitude of 353 miles and a speed of about 17,500 miles per hour. The Hubble Space Telescope (HST) transmits images and data from space to computers on Earth. In fact, HST sends enough data back to Earth each week to fill 3,600 feet of books on a shelf. Scientists store the data on special disks. In January 2006, HST captured images of the Orion Nebula, a huge area where stars are being formed. HST’s detailed images revealed over 3,000 stars that were never seen before. Information from the Hubble will help scientists understand more about how stars form. In this chapter, you will learn all about the star of our solar system, the sun, and about the characteristics of other stars. 1. Why do stars shine? 2. What kinds of stars are there? 3. How are stars formed, and do any other stars have planets? 16.1 The Sun and the Stars What are stars? Where did they come from? How long do they last? During most of the star - an enormous hot ball of gas day, we see only one star, the sun, which is 150 million kilometers away. On a clear held together by gravity which night, about 6,000 stars can be seen without a telescope. -

In-System'' Fission-Events: an Insight Into Puzzles of Exoplanets and Stars?
universe Review “In-System” Fission-Events: An Insight into Puzzles of Exoplanets and Stars? Elizabeth P. Tito 1,* and Vadim I. Pavlov 2,* 1 Scientific Advisory Group, Pasadena, CA 91125, USA 2 Faculté des Sciences et Technologies, Université de Lille, F-59000 Lille, France * Correspondence: [email protected] (E.P.T.); [email protected] (V.I.P.) Abstract: In expansion of our recent proposal that the solar system’s evolution occurred in two stages—during the first stage, the gaseous giants formed (via disk instability), and, during the second stage (caused by an encounter with a particular stellar-object leading to “in-system” fission- driven nucleogenesis), the terrestrial planets formed (via accretion)—we emphasize here that the mechanism of formation of such stellar-objects is generally universal and therefore encounters of such objects with stellar-systems may have occurred elsewhere across galaxies. If so, their aftereffects may perhaps be observed as puzzling features in the spectra of individual stars (such as idiosyncratic chemical enrichments) and/or in the structures of exoplanetary systems (such as unusually high planet densities or short orbital periods). This paper reviews and reinterprets astronomical data within the “fission-events framework”. Classification of stellar systems as “pristine” or “impacted” is offered. Keywords: exoplanets; stellar chemical compositions; nuclear fission; origin and evolution Citation: Tito, E.P.; Pavlov, V.I. “In-System” Fission-Events: An 1. Introduction Insight into Puzzles of Exoplanets As facilities and techniques for astronomical observations and analyses continue to and Stars?. Universe 2021, 7, 118. expand and gain in resolution power, their results provide increasingly detailed information https://doi.org/10.3390/universe about stellar systems, in particular, about the chemical compositions of stellar atmospheres 7050118 and structures of exoplanets. -

Hypothesis Paper Many Chemistries Could Be Used to Build Living
ASTROBIOLOGY Volume 4, Number 2, 2004 © Mary Ann Liebert, Inc. Hypothesis Paper Many Chemistries Could Be Used to Build Living Systems WILLIAM BAINS ABSTRACT It has been widely suggested that life based around carbon, hydrogen, oxygen, and nitrogen is the only plausible biochemistry, and specifically that terrestrial biochemistry of nucleic acids, proteins, and sugars is likely to be “universal.” This is not an inevitable conclusion from our knowledge of chemistry. I argue that it is the nature of the liquid in which life evolves that defines the most appropriate chemistry. Fluids other than water could be abun- dant on a cosmic scale and could therefore be an environment in which non-terrestrial bio- chemistry could evolve. The chemical nature of these liquids could lead to quite different biochemistries, a hypothesis discussed in the context of the proposed “ammonochemistry” of the internal oceans of the Galilean satellites and a more speculative “silicon biochemistry” in liquid nitrogen. These different chemistries satisfy the thermodynamic drive for life through different mechanisms, and so will have different chemical signatures than terrestrial biochemistry. Key Words: Carbon, hydrogen, oxygen, and nitrogen life—Planetary liquid— Silicon. Astrobiology 4, xxx–xxx. INTRODUCTION ISCUSSIONS of nonterrestrial life generally as- Dsume that the biochemistry of life will be similar to that which we see on Earth. Almost all There is a famous book published about writers assume that carbon is the central element 1912 by Lawrence J. Henderson . in which in any plausible biochemistry, and that its com- Henderson concludes that life necessarily bination with hydrogen, nitrogen, and oxygen is must be based on carbon and water, and the core of any living system. -

Production and Evolution of Li, Be and B Isotopes in the Galaxy
Astronomy & Astrophysics manuscript no. Prantzos˙Libeb2 c ESO 2018 July 18, 2018 Production and evolution of Li, Be and B isotopes in the Galaxy N.Prantzos1 Institut d’Astrophysique de Paris, UMR7095 CNRS, Univ.P. & M.Curie, 98bis Bd. Arago, 75104 Paris, France; e-mail: [email protected] ABSTRACT Context. We reassess the problem of the production and evolution of the light elements Li, Be and B and of their isotopes in the Milky Way in the light of new observational and theoretical developments. Aims. The main novelty is the introduction of a new scheme for the origin of Galactic cosmic rays (GCR), which for the first time enables a self-consistent calculation of their composition during galactic evolution. Methods. The scheme accounts for key features of the present-day GCR source composition, it is based on the wind yields of the Geneva models of rotating, mass-losing stars and it is fully coupled to a detailed galactic chemical evolution code. Results. We find that the adopted GCR source composition accounts naturally for the observations of primary Be and helps understanding why Be follows Fe more closely than O. We find that GCR produce ∼70% of the solar 11B/10B isotopic ratio; the remaining 30% of 11B presumably result from ν-nucleosynthesis in massive star explosions. We find that GCR and primordial nucleosynthesis can produce at most ∼30% of solar Li. At least half of the solar Li has to originate in low-mass stellar sources (red giants, asymptotic giant branch stars, or novae), but the required average yields of those sources are found to be much higher than obtained in current models of stellar nucleosynthesis.