Pacific Pocket Mouse (Perognathus Longimembris Pacificus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
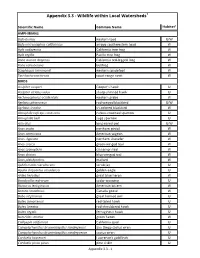
Appendix 3.3 - Wildlife Within Local Watersheds1
Appendix 3.3 - Wildlife within Local Watersheds1 2 Scientific Name Common Name Habitat AMPHIBIANS Bufo boreas western toad U/W Bufo microscaphus californicus arroyo southwestern toad W Hyla cadaverina California tree frog W Hyla regilla Pacific tree frog W Rana aurora draytonii California red-legged frog W Rana catesbeiana bullfrog W Scaphiopus hammondi western spadefoot W Taricha torosa torosa coast range newt W BIRDS Accipiter cooperi Cooper's hawk U Accipiter striatus velox sharp-shinned hawk U Aechmorphorus occidentalis western grebe W Agelaius phoeniceus red-winged blackbird U/W Agelaius tricolor tri-colored blackbird W Aimophila ruficeps canescens rufous-crowned sparrow U Aimophilia belli sage sparrow U Aiso otus long-eared owl U/W Anas acuta northern pintail W Anas americana American wigeon W Anas clypeata northern shoveler W Anas crecca green-winged teal W Anas cyanoptera cinnamon teal W Anas discors blue-winged teal W Anas platrhynchos mallard W Aphelocoma coerulescens scrub jay U Aquila chrysaetos canadensis golden eagle U Ardea herodius great blue heron W Bombycilla cedrorum cedar waxwing U Botaurus lentiginosus American bittern W Branta canadensis Canada goose W Bubo virginianus great horned owl U Buteo jamaicensis red-tailed hawk U Buteo lineatus red-shouldered hawk U Buteo regalis ferruginous hawk U Butorides striatus green heron W Callipepla californica California quail U Campylorhynchus brunneicapillus sandiegensis San Diego cactus wren U Campylorhynchus brunneicapillus sandiegoense cactus wren U Carduelis lawrencei Lawrence's -

Field Guide of Common Ants
J U L Y / A U G 2 0 0 3 N P M A L I B R A R Y U P D A T E Insert this update into the NPMA Pest Management Library, which can be Updatepurchased from the Resource Center. phone: 703-573-8330 fax: 703-573-4116 Common Ants: A Pull-Out Guide for Use in the Field This Library Update is designed to of acrobat ants. Workers are 1/16” to 1/8” assist technicians in identification and in length and are normally brown to control of ants while servicing accounts. black in color. The pedicel, or front joint This update is not designed for of the abdomen has two nodes or instruction in basic ant biology, segments. Looking down on the ant nomenclature of the anatomy of the ant, under hand lens magnification, one pair or to be used as a key. For more detailed of spines is found on the thorax. Various information on those topics, refer to the species of acrobat ants are found Field Guide or other technical materials. throughout the United States. In the field, a great aid to identification Acrobat ants are named for their is the use of a hand lens. Many of these tendency to raise their abdomens in the ants are small and positive identification air when disturbed. As the abdomen is is not easy without a hand lens. This heart shaped and is frequently black and article focuses on common, non-wood- shiny, the acrobat motion is readily destroying ants, including: acrobat, observable. -

Body Size, Not Phylogenetic Relationship Or Residency, Drives Interspecific Dominance in a Little Pocket Mouse Community
Animal Behaviour 137 (2018) 197e204 Contents lists available at ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/anbehav Body size, not phylogenetic relationship or residency, drives interspecific dominance in a little pocket mouse community * Rachel Y. Chock a, , Debra M. Shier a, b, Gregory F. Grether a a Department of Ecology & Evolutionary Biology, University of California, Los Angeles, CA, U.S.A. b Recovery Ecology, San Diego Zoo Institute for Conservation Research, Escondido, CA, U.S.A. article info The role of interspecific aggression in structuring ecological communities can be important to consider Article history: when reintroducing endangered species to areas of their historic range that are occupied by competitors. Received 6 September 2017 We sought to determine which species is the most serious interference competitor of the endangered Initial acceptance 6 November 2017 Pacific pocket mouse, Perognathus longimembris pacificus, and more generally, whether interspecific Final acceptance 1 December 2017 aggression in rodents is predicted by body size, residency status or phylogenetic relatedness. We carried out simulated territory intrusion experiments between P. longimembris and four sympatric species of MS. number: A17-00719 rodents (Chaetodipus fallax, Dipodomys simulans, Peromyscus maniculatus, Reithrodontomys megalotis)ina field enclosure in southern California sage scrub habitat. We found that body size asymmetries strongly Keywords: predicted dominance, regardless of phylogenetic relatedness or the residency status of the individuals. aggression The largest species, D. simulans, was the most dominant while the smallest species, R. megalotis, was the dominance least dominant to P. longimembris. Furthermore, P. longimembris actively avoided encounters with all interference competition Perognathus longimembris species, except R. -

Taxonomy and Distribution of the Argentine Ant, Linepithema Humile (Hymenoptera: Formicidae)
SYSTEMATICS Taxonomy and Distribution of the Argentine Ant, Linepithema humile (Hymenoptera: Formicidae) ALEXANDER L. WILD Department of Entomology, University of California at Davis, Davis, CA 95616 Ann. Entomol. Soc. Am. 97(6): 1204Ð1215 (2004) ABSTRACT The taxonomy of an invasive pest species, the Argentine ant, is reviewed. Linepithema humile (Mayr) 1868 is conÞrmed as the valid name for the Argentine ant. Morphological variation and species boundaries of L. humile are examined, with emphasis on populations from the antÕs native range in southern South America. Diagnoses and illustrations are provided for male, queen, and worker castes. Collection records of L. humile in South America support the idea of a native distribution closely associated with major waterways in lowland areas of the Parana´ River drainage, with recent intro- ductions into parts of Argentina, Brazil, Chile, Colombia, Ecuador, and Peru. KEY WORDS Argentine ant, Linepithema humile, taxonomy, invasive species THE ARGENTINE ANT, Linepithema humile (Mayr) 1868, MCSN, MCZC, MHNG, MZSP, NHMB, NHMW, and is among the worldÕs most successful invasive species. USNM; see below for explanation of abbreviations). This native South American insect has become a cos- Taxonomic confusion over L. humile extends be- mopolitan pest, particularly in the Mediterranean cli- yond museum collections. At least one important mates of North America, Chile, South Africa, Austra- study, seeking to explain Argentine ant population lia, and southern Europe (Suarez et al. 2001). regulation in the native range through phorid para- Argentine ants have been implicated in the decline of sitism (Orr and Seike 1998), initially targeted the native arthropod (Cole et al. 1992) and vertebrate wrong Linepithema species (Orr et al. -

Paleontological Resources of the Upper and Middle San Pedro Valley
Paleontological Resources of the Upper and Middle San Pedro Valley Robert D. McCord Arizona Museum of Natural History Geological setting Regional extension causing block faulting – creation of the Basin and Range ~15Ma Poorly developed drainage results in lakes in valley bottom ?-3.4 Ma Drainage develops with flow to north, marshes, ponds and lakes significant from time to time Early Pleistocene Saint David Formation ? – 3.4 million lakes, few fossils Well developed paleomagnetic timeframe – a first for terrestrial sediments! Succession of faunas from ~3 to 1.5 Ma Blancan to ? Irvingtonian NALMA Plants diatoms charophytes Equisetum (scouring rush) Ostracoda (aquatic crustaceans) Cypridopsis cf. vidua Limnocythere cf. staplini Limnocythere sp. Candona cf. renoensis Candona sp. A Candona sp. B ?Candonlella sp. ?Heterocypris sp. ?Cycloypris sp. Potamocypris sp. Cyprideis sp. Darwinula sp. Snails and a Clam Pisidium casertanum (clam) Fossaria dalli (aquatic snail) Lymnaea caperata (aquatic snail) Lymnaea cf. elodes (aquatic snail) Bakerilymnaea bulimoides (aquatic snail) Gyraulus parvus (aquatic snail) Promenetus exacuous (aquatic snail) Promenetus umbilicatellus (aquatic snail) Physa virgata (aquatic snail) Gastrocopta cristata (terrestrial snail) Gastrocopta tappaniana (terrestrial snail) Pupoides albilabris (terrestrial snail) Vertigo milium (terrestrial snail) Vertigo ovata (terrestrial snail) cf. Succinea (terrestrial snail) Deroceras aenigma (slug) Hawaila minuscula (terrestrial snail) Fish and Amphibians indeterminate small fish Ambystoma tigrinum (tiger salamander) Scaphiopus hammondi (spadefoot toad) Bufo alvarius (toad) Hyla eximia (tree frog) Rana sp. (leopard frog) Turtles and Lizards Kinosternon arizonense (mud turtle) Terrapene cf. ornata (box turtle) Gopherus sp. (tortoise) Hesperotestudo sp. (giant tortoise) Eumeces sp. (skink) “Cnemidophorus” sp. (whiptail lizard) Crotaphytus sp. (collared lizard) Phrynosoma sp. (horned lizard) Sceloporus sp. -

Draft Environmental Assessment of Marine Geophysical Surveys by the R/V Marcus G. Langseth for the Southern California Collaborative Offshore Geophysical Survey
DRAFT ENVIRONMENTAL ASSESSMENT OF MARINE GEOPHYSICAL SURVEYS BY THE R/V MARCUS G. LANGSETH FOR THE SOUTHERN CALIFORNIA COLLABORATIVE OFFSHORE GEOPHYSICAL SURVEY Submitted to: National Science Foundation Division of Ocean Sciences 4201 Wilson Blvd., Suite 725 Arlington, VA 22230 Submitted by: Scripps Institution of Oceanography, UCSD 8675 Discovery Way La Jolla, CA 92023 Contact: Professor Neal Driscoll 858.822.5026; [email protected] Prepared by: Padre Associates, Inc. 5290 Overpass Road, Suite 217 Goleta, CA 93113 June 2012 Southern California Collaborative Offshore Geophysical Survey (SCCOGS) Environmental Assessment TABLE OF CONTENTS 1.0 PURPOSE AND NEED ................................................................................................... 1 2.0 ALTERNATIVES INCLUDING PROPOSED ACTION ...................................................... 6 2.1 PROPOSED ACTION ......................................................................................... 6 2.2 PROJECT LOCATION ........................................................................................ 6 2.3 PROJECT ACTIVITIES ....................................................................................... 6 2.3.1 Mobilization and Demobilization .............................................................. 9 2.3.2 Offshore Survey Operations .................................................................... 9 2.3.2.1 Survey Vessel Specifications ..................................................... 10 2.3.2.2 Air Gun Description ................................................................... -

LOS ANGELES POCKET MOUSE Perognathus Longimembris Brevinasus
Terrestrial Mammal Species of Special Concern in California, Bolster, B.C., Ed., 1998 111 Los Angeles pocket mouse, Perognathus longimembris brevinasus Philip V. Brylski Description: This is a small heteromyid rodent, averaging about 113 mm TL with weight from 8 to 11 g. The Los Angeles pocket mouse can be potentially confused only with juveniles of the sympatric California pocket mouse (Chaetodipus californicus), from which it can be distinguished by the absence of spiny hairs in the dorsal pelage and the absence of a distinct crest on the tail. Pelage is buff above and white below. Many of the dorsal hairs are black-tipped, giving the pelage a "salt and pepper" appearance, similar to but lighter than that of the Pacific pocket mouse (P. l. pacificus). Like all silky pocket mice, there is usually a small white spot at the anterior base of the ear, and an indistinct larger buff spot behind the ear, the plantar surface of the hindfeet are naked or lightly haired, and the lateral hairs of the hind toes project anteriorly and laterally, resulting in a "fringed-toed" effect, which may enhance locomotor efficiency on sandy substrates. Taxonomic Remarks: This is one of eight subspecies of the little pocket mouse (P. longimembris) in California (Hall 1981). P. l. brevinasus was first described by Osgood (1900) as a race of the Panamint pocket mouse (P. panamintinus). Both brevinasus and panamintinus were arranged as subspecies of P. longimembris by Huey (1928). An important taxonomic character of brevinasus is its short rostrum, a character also shared by pacificus. P. -

The Case of the Argentine Ant in Vineyards of Northern Argentina
insects Article Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina Maria Schulze-Sylvester 1,* ID , José A. Corronca 1 ID and Carolina I. Paris 2 1 FCN-IEBI (Instituto para el Estudio de la Biodiversidad de Invertebrados), CONICET CCT-Salta, Facultad de Ciencias Naturales, Universidad Nacional de Salta, Av. Bolivia 5150, CP 4400 Salta, Argentina; [email protected] 2 Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Buenos Aires, 1428 Buenos Aires, Argentina; [email protected] * Correspondence: [email protected]; Tel.: +54-387-425-5472 Received: 10 December 2017; Accepted: 23 January 2018; Published: 29 January 2018 Abstract: The invasive Argentine ant causes ecological and economic damage worldwide. In 2011, this species was reported in vineyards of Cafayate, a wine-producing town in the Andes, Argentina. While the local xeric climate is unsuitable for Argentine ants, populations could establish in association with vineyards where human activity and irrigation facilitate propagule introduction and survival. In 2013–2014, we combined extensive sampling of the area using ant-baits with monitoring of the change in land use and vineyard cultivated area over the past 15 years. Our results revealed that the species has thus far remained confined to a relatively isolated small area, owing to an effective barrier of dry shrublands surrounding the infested vineyards; yet the recent expansion of vineyard acreage in this region will soon connect this encapsulated area with the rest of the valley. When this happens, vulnerable ecosystems and the main local industry will be put at risk. -

Breeding Biology and Diet of the Ferruginous Hawk in South Dakota
Wilson Bull., 94(l), 1982, pp. 4654 BREEDING BIOLOGY AND DIET OF THE FERRUGINOUS HAWK IN SOUTH DAKOTA CHARLES L. BLAIR AND FRANK SCHITOSKEY, JR. The Ferruginous Hawk (Buteo regalis) is the largest North American buteo. It occurs throughout most of the western United States and breeds from Alberta to western Texas and from Washington to Arizona. In this study we investigated the breeding biology of the Ferruginous Hawks in northwest South Dakota, complimenting earlier studies by Weston (1969) in Utah, Olendorff (1973) in Colorado, Howard (1975) in Utah and Idaho, and Lokemoen and Duebbert (1976) in north-central South Dakota. STUDY AREA AND METHODS The study area encompassed about 7000 km ’ in northwest South Dakota, including all of Harding County. The area is semi-arid and has a mid-continental climate with long, cold winters and short, warm summers (Spuhler et al. 1971). Eighty-five percent of Harding County is rangeland dominated by western wheatgrass (Agropyron smithii) and needle grass (Stipa comata). Sagebrush (Artemesia spp.) occurs throughout the area and is widespread in the western third of the county. Small grain crops compose 9% of the area, pastureland and tame hay 3% and woodland 3%. Elevated table lands are dominated by ponderosa pine (Pinw ponderma) Savannah, whereas green ash (Fraxinus pennsylvanicas), willow (S&x spp.) and Siberian elm (Ulmas pumila) predominate in riparian areas and ravines. Two or more biologists conducted a daily census of birds on the study area in 1976 and 1977; Ferruginous Hawk studies began the day the first hawk was sighted. Active nests were located either during aerial surveys at altitudes of 150-175 m, or by ground searches from roads. -

Recovery Research for the Endangered Pacific Pocket Mouse: an Overview of Collaborative Studies1
Recovery Research for the Endangered Pacific Pocket Mouse: An Overview of Collaborative Studies1 Wayne D. Spencer2 Abstract The critically endangered Pacific pocket mouse (Perognathus longimembris pacificus), feared extinct for over 20 years, was “rediscovered” in 1993 and is now documented at four sites in Orange and San Diego Counties, California. Only one of these sites is considered large enough to be potentially self-sustaining without active intervention. In 1998, I gathered a team of biologists to initiate several research tasks in support of recovery planning for the species. The PPM Studies Team quickly determined that species recovery would require active trans- locations or reintroductions to establish new populations, but that we knew too little about the biology of P. l. pacificus and the availability of translocation receiver sites to design such a program. Recovery research from 1998 to 2000 therefore focused on (1) a systematic search for potential translocation receiver sites; (2) laboratory and field studies on non-listed, surro- gate subspecies (P. l. longimembris and P. l. bangsi) to gain biological insights and perfect study methods; (3) studies on the historic and extant genetic diversity of P. l. pacificus; and (4) experimental habitat manipulations to increase P. l. pacificus populations. Using existing geographic information system (GIS) data, we identified sites throughout the historic range that might have appropriate soils and vegetation to support translocated P. l. pacificus. Re- connaissance surveys of habitat value were completed in all large areas of potential habitat identified by the model. Those sites having the highest habitat potential are being studied with more detailed and quantitative field analyses. -

US EPA-Pesticides; Disodium Octaborate Tetrahydrate
EFFICACY REVIEW DATE: IN 4-30-01 OUT 6-21-01 FILE OR REG. NO. 69529-1 PETITION OR EXP. PERMIT NO. DATE DIV. RECEIVED April 19, 2001 DATE OF SUBMISSION April 18, 2001 DATE SUBMISSION ACCEPTED TYPE PRODUCT(S): (I,)D, H, F, N, R, S DATA ACCESSION NO(S). 453568-01;D274488;S596027;Case#046539;AC:301 PRODUCT MGR. NO. 03-Layne/Quarles PRODUCT NAME(S) Pestbor COMPANY NAME Quality Borate Company SUBMISSION PURPOSE Provide performance data in reprint supporting claims for Argentine ant, pharaoh ant and Tap- inoma melanocephalum for formulated products. CHEMICAL & FORMULATION Disodium octaborate tetrahydrate 98% (30 lbs./cu.ft. bulk density manufacturing concentrate) CONCLUSIONS & RECOMMENDATIONS The data presented in EPA Accession (MRID) Number 453568-01, having been obtained from the reprint art- icle titled “Laboratory Evaluation of a Boric Acid Liquid Bait on Colonies of Tapinoma melanocephalum, Argentine Ants and Pharaoh Ants (Hymenoptera: Formicidae)S, which meets the requirements of § 11(b)(1)-(7) on p. 268 and the standard of § 95-11(c)(3)(b) on pp. 270-1 of the Product Performance Guidelines, are adequate to sup- port the registration of the subject product, the sole use for this formulation being for the manufacturing of end use insecticidal products, more specifically baits. The cited data (to be contin'd) is far more than is necessary to establish usefulness of this form- ulation for the making of insecticidal baits. Results with the 3 species included in the testing were as follows: Tapinoma melanocephalum workers were reduced by 97% -

Above-Belowground Effects of the Invasive Ant Lasius Neglectus in an Urban Holm Oak Forest
U B Universidad Autónoma de Barce lona Departamento de Biología Animal, de Biología Vegetal y de Ecología Unidad de Ecología Above-belowground effects of the invasive ant Lasius neglectus in an urban holm oak forest Tesis doctoral Carolina Ivon Paris Bellaterra, Junio 2007 U B Universidad Autónoma de Barcelona Departamento de Biología Animal, de Biología Vegetal y de Ecología Unidad de Ecología Above-belowground effects of the invasive ant Lasius neglectus in an urban holm oak forest Memoria presentada por: Carolina Ivon Paris Para optar al grado de Doctora en Ciencias Biológicas Con el Vº. Bº.: Dr Xavier Espadaler Carolina Ivon Paris Investigador de la Unidad de Ecología Doctoranda Director de tesis Bellaterra, Junio de 2007 A mis padres, Andrés y María Marta, y a mi gran amor Pablo. Agradecimientos. En este breve texto quiero homenajear a través de mi más sincero agradecimiento a quienes me ayudaron a mejorar como persona y como científica. Al Dr Xavier Espadaler por admitirme como doctoranda, por estar siempre dispuesto a darme consejos tanto a nivel profesional como personal, por darme la libertad necesaria para crecer como investigadora y orientarme en los momentos de inseguridad. Xavier: nuestras charlas más de una vez trascendieron el ámbito académico y fue un gustazo escucharte y compartir con vos algunos almuerzos. Te prometo que te enviaré hormigas de la Patagonia Argentina para tu deleite taxonómico. A Pablo. ¿Qué puedo decirte mi amor qué ya no te haya dicho? Gracias por la paciencia, el empuje y la ayuda que me diste en todo momento. Estuviste atento a los más mínimos detalles para facilitarme el trabajo de campo y de escritura.